Drinking a lot of decaf coffee is not environmentally friendly?
July 13 news, according to foreign media reports, the latest research by scientists shows that people like to drink low-caffeine coffee may damage the ozone layer. Decaf hot drinks contain a solvent, dichloromethane (dichloromethane), which, along with the massive production of dichloromethane, will delay the recovery of the ozone layer in Antarctica for 30 years.
Now scientists are calling for better control of dichloromethane, but the chemical is still found in decaf, hair styling agents, deodorants and a range of household cleaners. Ozone is a gas made up of three oxygen molecules, which can pose a threat to people's health near the ground, but ozone can protect human beings in the upper atmosphere and absorb ultraviolet radiation from the sun.
Dichloromethane is harmful because it floats upward and decomposes in the stratosphere close to the ozone layer. When the chemical breaks down, it destroys the surrounding molecules in the ozone layer, making the ozone hole larger and larger. As the ozone hole becomes larger, it will expose humans to the risk level of ultraviolet b radiation, which can tear up human DNA and cause carcinogenic mutations.
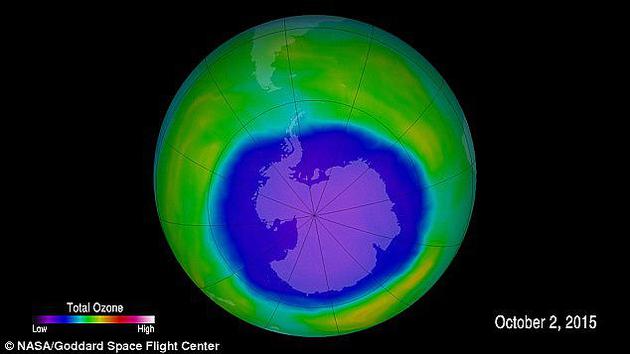
The picture shows the ozone layer over Antarctica. Purple and blue are the weakest areas of the ozone layer, while yellow and red are areas with more ozone.

Ozone is a gas composed of three oxygen molecules, which is potentially harmful to human health near the ground, but at the top of the atmosphere, ozone can play a protective role by absorbing ultraviolet radiation from the sun.
In the 1980s, scientists found that the unregulated use of chlorofluorocarbons and stable chlorine chemicals in household chemicals would gradually expand the ozone hole. In 1987, the United Nations Montreal Agreement banned the use of this harmful chemical, but dichloromethane, a volatile, ozone-depleting substance, was not banned.
Since the implementation of the Montreal Agreement, stratospheric ozone has begun to recover gradually, and the ozone index is expected to return to the level of the 1980s in the second half of this century. It is expected that the Antarctic "ozone hole" will be completely repaired in 2046-2057.
But now scientists warn that dichloromethane may play a role in ozone depletion and its use is increasing, such as decaf coffee containing dichloromethane. Dr Ryan Hossaini, a researcher at the University of Lancaster in the UK, said: "dichloromethane is a man-made, ozone-depleting chemical used in a range of industrial applications."
Unlike chlorofluorocarbons (CFCs) and similar stable gases, which cause ozone depletion, dichloromethane has a short atmospheric lifetime and is therefore not bound by the Montreal Agreement. Nevertheless, the increase in dichloromethane production has led to a rapid increase in atmospheric concentrations over the past decade.
Although there is not much ozone depletion due to dichloromethane at present, it is not clear how much the gas will be in the atmosphere in the future, which may pose a threat to ozone. "our results show that continued growth in dichloromethane concentrations will significantly delay the recovery of the ozone layer and offset the benign effects of the future Montreal Agreement," Hosani said.
Dr Stephen Montzka (Stephen Montzka) of the National Oceanic and Atmospheric Administration (NOAA) said: "the increase in the concentration of dichloromethane that we have measured is surprising and unexpected. The concentration of dichloromethane in the atmosphere began to rise slowly in the late 1990s, but the average concentration of dichloromethane in the global atmosphere tripled in early 2000."
Dr. Montezka stressed that the use of this dangerous chemical would potentially increase the use of chlorofluorocarbons banned in the 1980s. Professor Martin Chipfield (Martyn Chipperfield) of the Earth Environment Branch of the University of Leeds in the UK said that we need to continue to monitor the concentration of dichloromethane in the atmosphere and determine its source.
The current plan for long-term recovery of the ozone layer from the atmosphere under the influence of HCFCs is on the right track, but the increasing dichloromethane creates some uncertainties for us to predict the future ozone and climate. Dr. Hosani said that ozone is an important climate gas, it is affected by many factors, the gradual increase of dichloromethane will lead to ozone depletion, which is of great significance to the future climate impact. We should be aware of the threat posed by the gradual increase in dichloromethane and similar chemicals to stratospheric ozone, which are not bound by the Montreal Agreement and that much remains to be done to better understand and quantify the sources of dichloromethane in the atmosphere.
Important Notice :
前街咖啡 FrontStreet Coffee has moved to new addredd:
FrontStreet Coffee Address: 315,Donghua East Road,GuangZhou
Tel:020 38364473
- Prev

Britons who visit coffee shops every day to drink one cup a day may live an extra 9 minutes a day.
Follow the comments (Wechat official account vdailycom) found that the beautiful cafe opened a small shop of its own, Dr. Mark Gart, lead author of the International Agency for Research on Cancer, said that higher coffee consumption is associated with a lower risk of death from any cause, especially circulatory and digestive diseases.
- Next

New research suggests coffee increases life expectancy!
Weixin Official Accounts vdailycom Discover the Benefits of Opening Your Own Coffee Shop Previous USC studies have shown that coffee drinking is associated with several types of cancer, such as diabetes
Related
- Unexpected! Ruixing Telunsu lattes use a smoothie machine to foam milk?!
- % Arabia's first store in Henan opens into the village?! Netizen: Thought it was P's
- Does an authentic standard mocha coffee recipe use chocolate sauce or powder? Mocha Latte/Dirty Coffee/Salty Mocha Coffee Recipe Share!
- What is the difference between Vietnam egg coffee and Norway egg coffee? Hand-brewed single product coffee filter paper filter cloth filter flat solution!
- What is the difference between sun-cured and honey-treated coffee? What are the differences in the flavor characteristics of sun-honey coffee?
- How to make Italian latte! How much milk does a standard latte use/what should the ratio of coffee to milk be?
- How to make butter American/butter latte/butter Dirty coffee? Is hand-brewed coffee good with butter?
- Is Dirty the cold version of Australian White? What is the difference between dirty coffee/decent coffee and Australian white espresso?
- Relationship between brewing time and coffee extraction parameters How to make the brewing time fall to 2 minutes?
- Got entangled?! Lucky opens a new store, Mixue Ice City, and pursues it as a neighbor!