WTF? The damage to the ozone layer should be caused by the coffee pot?
For professional baristas, please follow the coffee workshop (Wechat official account cafe_style)

The decaf we like to drink will seriously damage the ozone layer over Antarctica, according to new research. The decaf we like to drink will seriously damage the ozone layer over Antarctica, according to new research.
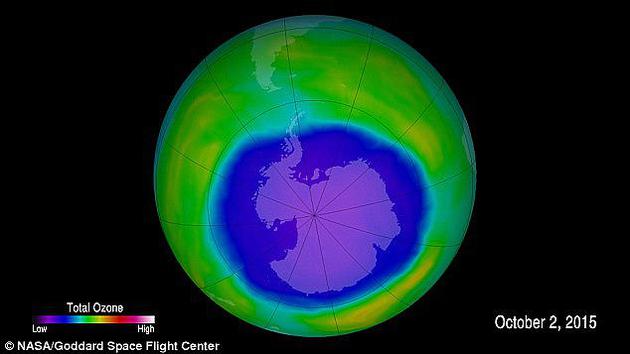
The picture shows the ozone layer over Antarctica. Purple and blue are the weakest areas of the ozone layer, while yellow and red are areas with more ozone. The picture shows the ozone layer over Antarctica. Purple and blue are the weakest areas of the ozone layer, while yellow and red are areas with more ozone.

Ozone is a gas composed of three oxygen molecules, which is potentially harmful to human health near the ground, but at the top of the atmosphere, ozone can play a protective role by absorbing ultraviolet radiation from the sun. Ozone is a gas composed of three oxygen molecules, which is potentially harmful to human health near the ground, but at the top of the atmosphere, ozone can play a protective role by absorbing ultraviolet radiation from the sun.
Beijing, July 13 (Xinhua) according to foreign media reports, the latest research by scientists shows that people's favorite low-caffeine coffee may damage the ozone layer. Decaf hot drinks contain a solvent, dichloromethane (dichloromethane), which, along with the massive production of dichloromethane, will delay the recovery of the ozone layer in Antarctica for 30 years. WTF? The damage to the ozone layer should be caused by the coffee pot?
Now scientists are calling for better control of dichloromethane, but the chemical is still found in decaf, hair styling agents, deodorants and a range of household cleaners. Ozone is a gas made up of three oxygen molecules, which can pose a threat to people's health near the ground, but ozone can protect human beings in the upper atmosphere and absorb ultraviolet radiation from the sun.
Dichloromethane is harmful because it floats upward and decomposes in the stratosphere close to the ozone layer. When the chemical breaks down, it destroys the surrounding molecules in the ozone layer, making the ozone hole larger and larger. As the ozone hole becomes larger, it will expose humans to the risk level of ultraviolet b radiation, which can tear up human DNA and cause carcinogenic mutations.
In the 1980s, scientists found that the unregulated use of chlorofluorocarbons and stable chlorine chemicals in household chemicals would gradually expand the ozone hole. In 1987, the United Nations Montreal Agreement banned the use of this harmful chemical, but dichloromethane, a volatile, ozone-depleting substance, was not banned.
Since the implementation of the Montreal Agreement, stratospheric ozone has begun to recover gradually, and the ozone index is expected to return to the level of the 1980s in the second half of this century. It is expected that the Antarctic "ozone hole" will be completely repaired in 2046-2057.
But now scientists warn that dichloromethane may play a role in ozone depletion and its use is increasing, such as decaf coffee containing dichloromethane. Dr Ryan Hossaini, a researcher at the University of Lancaster in the UK, said: "dichloromethane is a man-made, ozone-depleting chemical used in a range of industrial applications."
Unlike chlorofluorocarbons (CFCs) and similar stable gases, which cause ozone depletion, dichloromethane has a short atmospheric lifetime and is therefore not bound by the Montreal Agreement. Nevertheless, the increase in dichloromethane production has led to a rapid increase in atmospheric concentrations over the past decade.
Although there is not much ozone depletion due to dichloromethane at present, it is not clear how much the gas will be in the atmosphere in the future, which may pose a threat to ozone. "our results show that continued growth in dichloromethane concentrations will significantly delay the recovery of the ozone layer and offset the benign effects of the future Montreal Agreement," Hosani said.
Dr Stephen Montzka (Stephen Montzka) of the National Oceanic and Atmospheric Administration (NOAA) said: "the increase in the concentration of dichloromethane that we have measured is surprising and unexpected. The concentration of dichloromethane in the atmosphere began to rise slowly in the late 1990s, but the average concentration of dichloromethane in the global atmosphere tripled in early 2000."
Dr. Montezka stressed that the use of this dangerous chemical would potentially increase the use of chlorofluorocarbons banned in the 1980s. Professor Martin Chipfield (Martyn Chipperfield) of the Earth Environment Branch of the University of Leeds in the UK said that we need to continue to monitor the concentration of dichloromethane in the atmosphere and determine its source.
The current plan for long-term recovery of the ozone layer from the atmosphere under the influence of HCFCs is on the right track, but the increasing dichloromethane creates some uncertainties for us to predict the future ozone and climate. Dr. Hosani said that ozone is an important climate gas, it is affected by many factors, the gradual increase of dichloromethane will lead to ozone depletion, which is of great significance to the future climate impact. We should be aware of the threat posed by the gradual increase in dichloromethane and similar chemicals to stratospheric ozone, which are not bound by the Montreal Agreement and that much remains to be done to better understand and quantify the sources of dichloromethane in the atmosphere.
Important Notice :
前街咖啡 FrontStreet Coffee has moved to new addredd:
FrontStreet Coffee Address: 315,Donghua East Road,GuangZhou
Tel:020 38364473
- Prev

Can raw coffee beans sprout? Passengers carrying 60 kilograms of raw coffee beans into the country were returned.
Can raw coffee beans sprout? Passengers carrying 60 kilograms of raw coffee beans into the country were returned to professional baristas for communication. Recently, staff of Shandong Weihai entry-exit Inspection and Quarantine Bureau found that a passenger from Incheon, South Korea, attempted to bring large quantities of raw coffee beans into the country illegally during quarantine inspection at the seaport port. According to investigation, the batch
- Next

What is a single cup of coffee?
Following Cafe Review (Wechat official account vdailycom) found that Beautiful Cafe opened a small shop of its own. What is an individual coffee? As the name implies, it is a single variety of coffee. Coffee beans produced in a certain area are coffee made from coffee powder ground from a single type of coffee rather than mixed coffee, that is, single-product coffee. And individual coffee is usually not served with coffee companions.
Related
- What grade does Jamaica Blue Mountain No. 1 coffee belong to and how to drink it better? What is the highest grade of Blue Mountain coffee for coffee aristocrats?
- What are the flavor characteristics of the world-famous coffee Blue Mountain No. 1 Golden Mantelin? What are the characteristics of deep-roasted bitter coffee?
- Can I make coffee a second time in an Italian hand-brewed mocha pot? Why can't coffee be brewed several times like tea leaves?
- Hand-brewed coffee flows with a knife and a tornado. How to brew it? What is the proportion of grinding water and water temperature divided into?
- What is the difference between Indonesian Sumatra Mantinin coffee and gold Mantinin? How to distinguish between real and fake golden Mantelin coffee?
- What does bypass mean in coffee? Why can hand-brewed coffee and water make it better?
- Unexpected! Ruixing Telunsu lattes use a smoothie machine to foam milk?!
- % Arabia's first store in Henan opens into the village?! Netizen: Thought it was P's
- Does an authentic standard mocha coffee recipe use chocolate sauce or powder? Mocha Latte/Dirty Coffee/Salty Mocha Coffee Recipe Share!
- What is the difference between Vietnam egg coffee and Norway egg coffee? Hand-brewed single product coffee filter paper filter cloth filter flat solution!

