Ozone layer damaged low-caffeine back pot? How is decaf made?
For professional baristas, please follow the coffee workshop (Wechat official account cafe_style)
According to foreign media reports, the latest research by scientists shows that people like to drink low-caffeine coffee may damage the ozone layer. Decaf uses a solvent, dichloromethane (dichloromethane), which, along with the massive production of dichloromethane, will delay the recovery of the ozone layer in Antarctica for 30 years.
WTF? The damage to the ozone layer should be caused by the coffee pot?

Now scientists are calling for better control of dichloromethane, but the chemical is still found in decaf, hair styling agents, deodorants and a range of household cleaners.
Dichloromethane is harmful because it floats upward and decomposes in the stratosphere close to the ozone layer. When the chemical breaks down, it destroys the surrounding molecules in the ozone layer, making the ozone hole larger and larger. As the ozone hole becomes larger, it will expose humans to the risk level of ultraviolet b radiation, which can tear up human DNA and cause carcinogenic mutations.

Ozone is a gas made up of three oxygen molecules, which can pose a threat to people's health near the ground, but ozone can protect human beings in the upper atmosphere and absorb ultraviolet radiation from the sun.
In the 1980s, scientists found that the unregulated use of chlorofluorocarbons and stable chlorine chemicals in household chemicals would gradually expand the ozone hole. In 1987, the United Nations Montreal Agreement banned the use of this harmful chemical, but dichloromethane, a volatile, ozone-depleting substance, was not banned.
Since the implementation of the Montreal Agreement, stratospheric ozone has begun to recover gradually, and the ozone index is expected to return to the level of the 1980s in the second half of this century. It is expected that the Antarctic "ozone hole" will be completely repaired in 2046-2057.
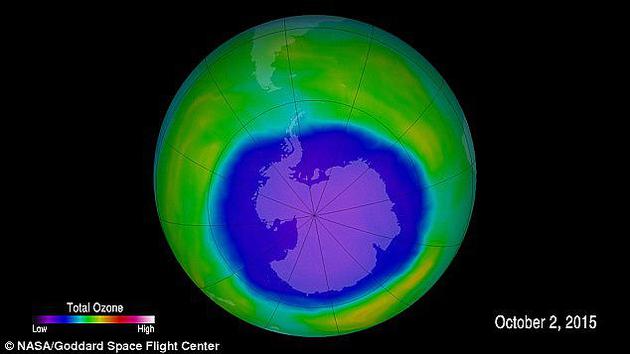
The picture shows the ozone layer over Antarctica. Purple and blue are the weakest areas of the ozone layer, while yellow and red are areas with more ozone.
But scientists warn that the industrial use of dichloromethane is increasing. Unlike chlorofluorocarbons (CFCs) and similar stable gases that deplete ozone, dichloromethane has only a short atmospheric lifetime and is therefore not subject to the Montreal Agreement. But even so, the increase in dichloromethane has led to a rapid increase in atmospheric concentrations over the past decade.
Although there is not much ozone depletion caused by dichloromethane at present, it is not clear how much the gas will be in the atmosphere in the future and how much threat it will pose to ozone. However, scientists still believe that the continued increase in the concentration of dichloromethane will significantly delay the recovery of the ozone layer and offset the benign effects of the future Montreal Agreement.
NOAA measurements found that atmospheric dichloromethane concentrations began to rise slowly in the late 1990s and tripled the global average atmospheric dichloromethane concentration at the beginning of 2000.
Professor Martin Chipfield (Martyn Chipperfield) of the Earth Environment Branch of the University of Leeds in the UK said that we need to continue to monitor the concentration of dichloromethane in the atmosphere and determine its source.
……
Wait, wait, wait...
Are you trying to knock me out?
To take away my coffee beans?

Dichloromethane is harmful
Dichloromethane destroys the ozone layer
Increased concentration of dichloromethane
Yeah, okay. And?
Dichloromethane off caffeine coffee what's the matter?
Popular science time
What is decaf?
In general, Arabica coffee beans contain 1.1%-1.7% caffeine, while robusta beans contain 2%-4.5% d caffeine. Decaf coffee is required to contain no more than 0.3% caffeine in brewed coffee. That means no more than 5 milligrams of caffeine in a cup of decaf.
Decaffeinated coffee actually eliminates about 97% of the caffeine, not a complete cause.

How is decaf produced?
The steps of low-cause treatment can only be carried out in the state of raw coffee beans.
Today, there are three main types of treatments to remove caffeine: traditional / European treatment (European Process), Swiss water treatment (SWP,Swiss Water Process), and CO supercritical Process (CO2 supercritical treatment). All three methods are very effective in removing most caffeine, leaving only 20.3% of the original caffeine in coffee beans, but more or less, it will affect the flavor of the coffee itself.

Dichloromethane occurs only in traditional / European treatment (European Process), and dichloromethane (CH 2 Cl 2) is the most common method of decaffeination, accounting for 50-75% of the decaf market.
In the European approach, there are two subdivisions:
Direct solvent (dichloromethane) method. Steam opens the hole of raw beans, sprays caffeine solvent directly, and continues to remove solvent and caffeine by "steaming".
Indirect solvent (dichloromethane) method. Soak the raw beans in hot water and soak everything you can. Separate the water beans. Use the solvent to remove the caffeine from the hot water and spray the rest of the "taste" back onto the tasteless bean shell.
Swiss water treatment: the first batch of raw bean bubble hot water, after the soluble substances are dissolved in the water, the beans are thrown away. The caffeine in the water is removed by carbon filter, leaving the "flavor-saturated liquid". After that, add raw beans, only caffeine will dissolve, and the "flavors" will remain in the beans (solubility problem). What's more, as long as the carbon is filtered again, the "flavor saturation" after caffeine removal can continue to be used.

Before brushing a wave of transparent coffee on the screen, professionals speculate that it should be decolorized by a method similar to that of Swiss water treatment.
The last method is powerful just by hearing the name, which is called "supercritical carbon dioxide high pressure extraction". Producers will add high-pressure liquid carbon dioxide to coffee beans. Under high pressure, carbon dioxide is in a semi-gaseous and semi-liquid state, in which carbon dioxide can be actively combined with caffeine and then converted to gaseous release, which can take away caffeine, which is more expensive, but has relatively little impact on the taste of coffee.

The disadvantages of decaf coffee
Low-reason coffee production process is complex, the output is generally not high, and now the market will be out of stock from time to time. However, decaf coffee is not rare and expensive, its processing cost is only 5% Mel 10% higher than ordinary coffee beans, and the market acceptance is not high, so the price of decaf coffee and ordinary coffee is usually about the same. Low-caffeine coffee beans go through more processing procedures than normal coffee beans, so they are more likely to oxidize and deteriorate, and the sealed shelf life of low-caffeine coffee beans is 30 seconds and 60 days less than that of regular coffee beans.
Without caffeine, the flavor of coffee must decline, especially for coffee beans that use chemical methods to "decaffeinate". Apart from the mature illy technology of Italian Coffee, the founder of Italian Coffee, few can control this technology well, and it is inevitable that there are residues of chemical solvent dichloromethane, which affects the flavor of coffee beans.

No one likes such an unrefreshing coffee with a discounted flavor, except for a few special groups, and even in the United States, less than 10 percent of people choose decaf, according to the American Coffee Association. The American Heart Association also announced that studies have found that decaf coffee may increase the body's low density lipoprotein cholesterol and may affect heart health.

Caffeine helps break down cholesterol in the body. Decaffeinated coffee does not have this effect because it contains very little caffeine. If you drink it with sugar and milk, it will become an unhealthy drink. Decaf coffee has a lighter flavor than ordinary coffee. if you drink decaf coffee for the sake of health, you might as well choose milk-free and sugar-free "fasting coffee".
Do you have all-natural decaf?
The answer may disappoint you, yes, but the output is very small, and the price is too expensive.

With a caffeine content of only 0.6%, it is the only natural low-caffeine coffee in the world.
Pointed Bourbon is a variety derived from Reunion Island. the normal bourbon variety has short round beans, but in 1810 Rayloy, a coffee farmer on Bourbon Island, found that there was a short coffee in the garden, with smaller leaves, shaped like laurel leaves, and leaner beans with less yield than ordinary bourbon. Botanists confirmed that it was a new variety at that time, so they named her after the name of the person who found her. Also because the two ends of the bean body are very sharp, it is commonly known as the pointed body bourbon.
In addition to its unique appearance, the pointed Bourbon has some unique characteristics that make it a unique breed. She is a natural semi-decaf coffee with only half the caffeine content of ordinary Arabica, the common Arabica caffeine content of about 1.2%, sharp body bourbon only 0.6%, but mellow, with rich fruit notes.

Another characteristic of the pointed bourbon is its low production capacity, which is much lower than that of other coffee varieties, and is extremely vulnerable to disease caused by the external environment. For this reason, farmers in Bourbon Island gave up coffee in 1950 to plant more profitable sugarcane, so that the bourbon round and pointed bodies on the island were once considered extinct. It was not until 2001 that Shodai Kawajima, an expert on UCC coffee in Japan, got the confirmation that the pointed bourbon still existed. And joined hands with botanists from the French International Agricultural Development Research Center to restore semi-hypogenic pointed bourbon. In 2006, the first batch of reborn pointed bourbon was harvested. 700kg pointed beans were produced, and 240kg were shipped to Japan. The coffee was roasted and packed into a pocket bag of 100g per unit, which cost 7350 yen and was robbed in one day. In 2011, the price rose to 8400 yen per 100g, which is the noblest coffee today.
Important Notice :
前街咖啡 FrontStreet Coffee has moved to new addredd:
FrontStreet Coffee Address: 315,Donghua East Road,GuangZhou
Tel:020 38364473
- Prev

Popular science | what is coffee blending? Why is it so delicious?
The exchange of professional baristas please follow the coffee workshop (Wechat official account cafe_style) mixed coffee, also known as mixed coffee roaster to understand the characteristics of each kind of coffee beans, and artistically mix them to create a desired new flavor. The knowledge of roasters mixing coffee beans can be said to be the highest industry secret. Almost all the popular Italian styles nowadays belong to
- Next

The proportion of Italian beans, Italian beans is not so complicated.
Following Cafe (Wechat official account vdailycom) found that it is very important for every baker to open a small shop in Beautiful Cafe and understand the complexity of Italian concentrate matching. This is not only their housekeeping skill, but also their exclusive secret recipe. It can be said that the matching formula determines the brand image of a coffee shop. However, because the formula is not protected by intellectual property rights, every
Related
- Guji coffee producing area of Guji, Ethiopia: Humbela, Shakiso, Wulaga
- What is the most expensive variety of Qiloso in BOP multi-variety group?
- How to store the coffee beans bought home?
- Why are Yemeni coffee beans so rare now?
- Ethiopian Sidamo all Red Fruit Sun Sun Santa Vini Coffee beans
- SOE is mostly sour? What does it mean? Is it a single bean? what's the difference between it and Italian blending?
- Is Italian coffee beans suitable for making hand-brewed coffee?
- How to choose coffee beans when making cold coffee? What kind of coffee beans are suitable for making cold coffee?
- Just entered the pit to make coffee, what kind of coffee beans should be chosen?
- Can only Japan buy real Blue Mountain Coffee? What are authentic Jamaican Blue Mountain coffee beans?

