How to produce decaf coffee? Solvent extraction supercritical fluid extraction water treatment

Having a cup of coffee in the morning to kick off the day's work has become a daily ritual for many people, and even tasting coffee has become a social activity. However, the problem that bothers many people is that although they like the unique smell of coffee, they do not want coffee to be too "refreshing" to affect their daily life, and the market for decaffeinated coffee (decaf coffee, also known as decaf coffee) is born! But how on earth did such a cup of coffee come into being?
Decaf is not a demand for coffee in the 21st century.
The refreshing effect of caffeine can be said to be the main reason why people can't do without it. Caffeine is a central nervous system stimulant, caffeine intake can make people feel less tired, as well as improve attention, reduce pain and other effects.
However, caffeine is slightly addictive, making it less and less refreshing, eventually leading to overintake, which can cause palpitations or interfere with sleep. This is why decaf coffee has become the choice of more and more people, consumers can not only moderate intake of caffeine, but also enjoy the aroma of coffee.
As a matter of fact, decaffeinated coffee is not a new demand in the 21st century. As early as the beginning of the 20th century, Kaffee Hag, the first company in the world to sell decaffeinated coffee (later acquired and renamed Caf é Hag by American merchants), developed this product.
It's just that not many people drank decaf at that time. Until the end of World War II, ── Robusta; Coffea canephora, a coffee bean with high caffeine content, rose. However, due to the heavy use of Luodou, many people can not stand high concentrations of caffeine, increasing the demand for decaf coffee.
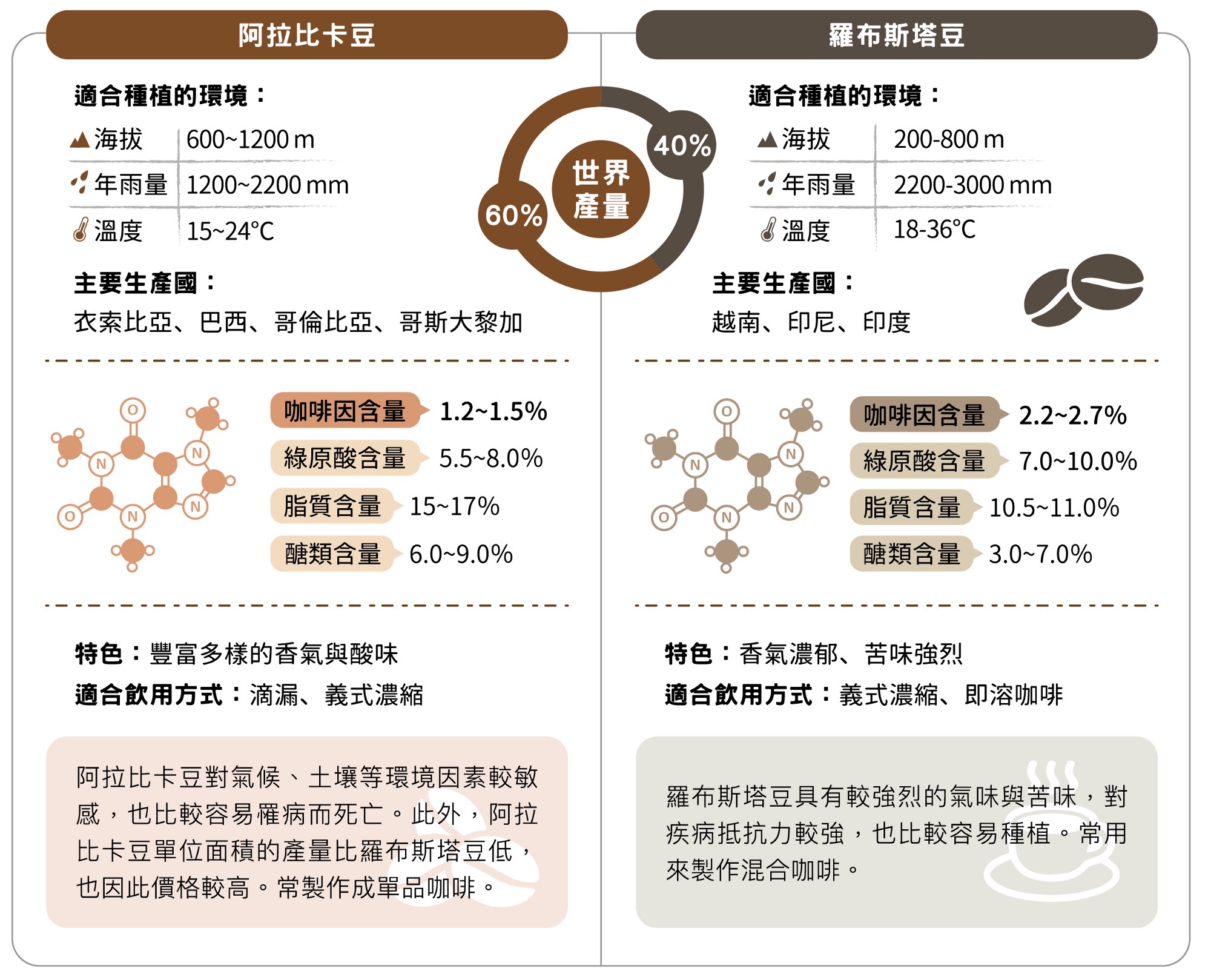
Most of the common coffee in our life comes from Coffea arabica, which accounts for about 60% of the global production. Because the coffee brewed has a variety of aroma and rich sour taste, it is mostly made into a variety of boutique coffee.
Robusta beans, on the other hand, have a strong smell and bitter taste, so they are often mixed or instant coffee, with a global production of nearly 40%. In addition, Arabica beans contain about 1.2% to 1.5% caffeine, while Robbosa beans are nearly twice as high, about 2.2% to 2.7%.
After World War II, more and more coffee products made from Luodou were used, but because of the high caffeine content, it was easy for consumers to feel uncomfortable, so many manufacturers began to invest resources to develop decaf coffee products.
Removal of caffeine by extraction
The process of decaf is mostly a trade secret of various companies, but we can still know the common ways of making decaf from the published literature and patents, but the details are not known.
The common way to remove caffeine is to use "extraction". Generally speaking, raw coffee beans are extracted to remove caffeine before drying and baking. The common extraction methods can be divided into three categories: solvent extraction (solvent decaffeination), supercritical fluid extraction (CO fluid decaffeination) and water treatment.
Solvent extraction method
Before the 1970s, low-caffeine coffee sold in commercial quantities only used solvent extraction. Until then, scientists did not have a clear understanding of the effects of various solvents on the human body, so many solvents now thought to have health concerns or cancer risks have been used by manufacturers to extract caffeine, including benzene, chloroform and carbon tetrachloride, but the most common extraction solvents are dichloromethane and ethyl acetate.
According to the different treatment steps, solvent extraction can be divided into "direct solvent extraction" and "indirect solvent extraction". Because caffeine binds to chlorogenic acid (Chlorogenic acid, CGA) in beans, it is necessary to separate caffeine molecules from dried coffee beans by absorbing enough water before starting extraction.
The direct extraction method is to directly steam the dried raw coffee beans and soak them in the solvent. After the caffeine is dissolved, the raw beans are removed and heated to volatilize the solvent and water.
The indirect extraction rule is to boil the raw beans in hot water, dissolve caffeine and various odor molecules, and then fully mix the boiled coffee beans with the solvent to let the caffeine dissolve into the solvent. after removing the solvent, rinse the raw beans with the remaining water so that the odor molecules can return to the beans, and then drain and dry.
When it comes to caffeine extraction solvents, dichloromethane dominated the decaf industry before the 1970s and is recognized as the best solvent to use. In addition to the quite good extraction effect, its colorless, boiling point 39.6℃ is easy to volatilize and non-flammable, which makes the process quite effective and safe. But ironically, dichloromethane was eventually abandoned not because it was found to be carcinogenic, but because it was suspected to be one of the culprits of the hole in the ozone layer.
When dichloromethane was out of fashion, it was replaced by ethyl acetate. Ethyl acetate will occur naturally in nature, and many fruits themselves contain a certain proportion, so there is no doubt about safety. In 1982, the U.S. Food and Drug Administration (FDA) approved ethyl acetate for caffeine removal, but because many foods contain ethyl acetate, it is impossible to directly explore its health effects, so there is no clear licensing standard for residues.
Supercritical fluid extraction
The disadvantage of solvent extraction is that the treated coffee beans will seriously lose their aroma and flavor, so some manufacturers try to develop selective extraction methods, trying to take away only caffeine and leave a good taste. The supercritical fluid method was patented in the United States by Cafe Hag in the early 1970s.
The new method developed by the industry is to successfully dissolve only caffeine from water-saturated raw beans by using carbon dioxide supercritical fluid as a solvent in a tank at about 300atmospheric pressure (atm) and 65 ℃. The supercritical fluid of carbon dioxide flows into the water of another tank with caffeine, and the lower temperature and pressure in the tank will make the carbon dioxide return to gaseous state, so that the caffeine can be smoothly transferred to the water, and the carbon dioxide can be recycled back to the high temperature and high pressure tank. This process lasts for 8 to 12 hours to reduce the caffeine content in raw beans to about 0.08%.
The advantage of using carbon dioxide supercritical fluid is that its physical properties are between liquid and gaseous state, and its viscosity is close to that of gas, so it consumes less energy than when transporting liquid, and its density is close to that of liquid. therefore, more solvent molecules can be transported per unit time than gas. Since almost only caffeine is dissolved, more odor molecules can be retained in raw beans, and as long as you return to normal temperature and pressure, carbon dioxide will return to gaseous state and escape, which is safer than the above-mentioned solvent.
However, the disadvantage of supercritical extraction is that the facilities invested at the initial stage are extremely expensive and not easy to get back to cost, so only a small number of well-capitalized enterprises can use this technology.
Water treatment method
In addition to developing the expensive supercritical fluid method, another group of people developed a way to extract caffeine with water as a solvent without losing the smell of coffee. This method is much cheaper and easier to operate than the supercritical fluid method, and the most common water treatment method is the Swiss Water treatment (Swiss water process) developed by the Swiss company Coffex in the late 1970s.

In Swiss water treatment, a batch of raw beans are soaked in hot water to dissolve caffeine and odor molecules, then water is introduced into activated carbon to absorb caffeine, and then the filtered water that still contains odor molecules will continue to be used in the extraction process of the next batch of raw beans.
The next batch of raw beans can be successfully retained in the beans because the water used for extraction contains saturated odor molecules, so only caffeine will be dissolved and odor molecules will be dissolved. In addition to the lower cost, this method is safer to use water as solvent than organic solvent, so it is also the method used by many low-quality coffee at present.
Important Notice :
前街咖啡 FrontStreet Coffee has moved to new addredd:
FrontStreet Coffee Address: 315,Donghua East Road,GuangZhou
Tel:020 38364473
- Prev

The secret of a perfect Espresso? Better, cheaper, greener Espresso?
Nowadays, most of the drinks in boutique coffee shops are based on Espresso coffee, whether it is Americano, Latte or a cup of Espresso Martini, they are all based on Espresso. However, cooking a cup of Espresso is also the most complicated, which involves too many combinations and influencing factors, such as the choice of coffee beans and grinding.
- Next

The difference between deep roasting and light roasting of coffee beans how is the flavor and taste of light roasted coffee
There is really a lot of knowledge about coffee, just like the fresh ingredients in the hands of chefs, which change more or less from season to season, but generally speaking, the way coffee is cooked is to roast raw beans to the degree desired by the baker. weaken the unwanted flavor and strengthen the flavor you like. Therefore, there are many problems with the depth of baking.
Related
- Beginners will see the "Coffee pull flower" guide!
- What is the difference between ice blog purified milk and ordinary milk coffee?
- Why is the Philippines the largest producer of crops in Liberia?
- For coffee extraction, should the fine powder be retained?
- How does extracted espresso fill pressed powder? How much strength does it take to press the powder?
- How to make jasmine cold extract coffee? Is the jasmine + latte good?
- Will this little toy really make the coffee taste better? How does Lily Drip affect coffee extraction?
- Will the action of slapping the filter cup also affect coffee extraction?
- What's the difference between powder-to-water ratio and powder-to-liquid ratio?
- What is the Ethiopian local species? What does it have to do with Heirloom native species?

