Professional coffee roasting| Can Taguchi make mistakes? Glass conversion temperature and coffee bitterness
For professional baristas, please follow the coffee workshop (Wechat official account cafe_style)
Usually, the more serious people are, the easier it is to get stuck in the mud, and the harder they are, the deeper they get. As for the discussion of the causes of the bitterness of coffee, the mainstream way of saying is to blame it on chlorogenic acid, but there are not many works on coffee in China. Mr. Taguchi's translation is a very important source of relevant knowledge, especially in the Coffee equation in which he cooperated with Danbu. Taguchi no longer only talks about Taguchi's point of view, but with the theoretical support of Danbu, that book can be said to be very practical and valuable. However, it is reasonable to say that the morphological transformation in the process of coffee roasting and heating, that is, the so-called transformation of glassy state and rubber state, is linked to the chemical reaction of chlorogenic acid, which in turn affects whether the flavor of coffee is bitter or not. Not to mention how to achieve it in practice.
I've been hanging around on @ atti @ bug since I had friends there last night. I think it's necessary to try to sort out the information I collected and my own thoughts again. I hope it will be of some help to you.
Taguchi uses a simple dichotomy to express the heating reaction of chlorogenic acid in Mena stage. First, decomposition (hydrolysis) with water will produce quinic acid (sour) and caffeic acid (bitter). Further heating of caffeic acid will turn into vinyl catechol polymer (bad bitterness). Second, it is dehydrated and condensed into chlorogenic acid lactone (good bitter taste). When the coffee is in a rubber state with high water content, it is easy to decompose chlorogenic acid with water, so Mr. Tian advocated that it should go through the rubber zone as soon as possible when baking, but did not say how to do it to achieve its goal, so the believers had to rely on their own skills.
The so-called glassy / rubber state of coffee refers to the physical change process of coffee beans from hard (glassy) to soft and elastic (rubber) > hard (glassy) due to changes in temperature and water content during coffee roasting and heating.

Source: www.probatburns.com
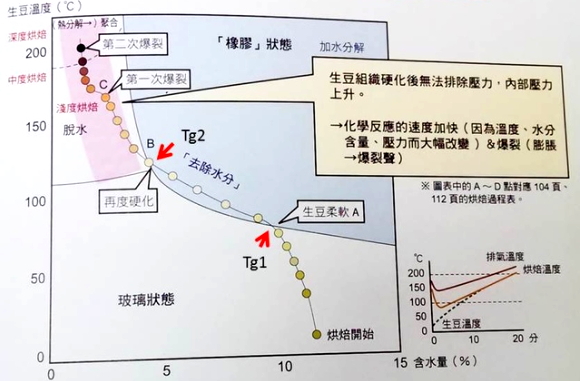
The above picture cites the study of Schenker and Geiger, where the solid black line sliding from top left to right represents the boundary between the glassy state and the rubber state of coffee beans at different core temperatures and moisture contents, known as glass transition temperature (Glass transition temperature). In stage (1), the core temperature of the bean is lower than the glass transition temperature, so most of the tissue of the bean is in the hard glassy state; in stage (2), the core temperature of the bean is higher than the glass transition temperature, so most of the tissue of the bean is in a soft and elastic rubber state, and at the same time, due to the pressure formed by internal water vapor and other gases, the bean at this stage has the highest expansion rate. In stage 3, the core temperature of the bean is lower than the glass transition temperature again, at this time the moisture content of the bean is very low, and most of the tissue is in the dry and hard glass state.
Since the diagram expresses the relationship between the core temperature and moisture content of beans in a two-dimensional way, without taking into account the baking time, the red line with the left arrow on the picture is trying to express the path of the transition of beans between the glassy state and the rubber state during baking. the larger the area framed by the red line and glass conversion temperature, the greater the proportion of beans in the rubber state during the baking process, which can be inferred from this. The higher the proportion of chlorogenic acid decomposed by adding water.
The picture above is very clear, but it may be misleading to mark the specific figures, and so are the illustrations in Taguchi's book, because their numbers are a long way from everyone's operational experience.
Let's take a look at the source of the literature they quoted.

Literature source

Fig 33 above is the literature source of the drawings in probatburns and Taguchi. Have you noticed that this is a research paper by an official Swiss research institute, which does not show the figures of temperature and moisture content, which means that this is a schematic diagram, not the result of experimental measurements, which proves that the figures marked on it are unreliable.
In Fig 33, there are two baking modes: LTLT (slow baking at low temperature) and HTST (fast baking at high temperature). We found that the area of rubber state occupied by HTST is larger than that of LTLT, implying that in HTST mode, the proportion of chlorogenic acid decomposing with water should be higher. But the total baking time of HTST (high temperature fast baking) is only 180s, which is much lower than the 720s of LTLT (slow baking at low temperature). Isn't it faster to pass through the rubber state? How can the proportion of decomposition reaction by adding water be relatively high?
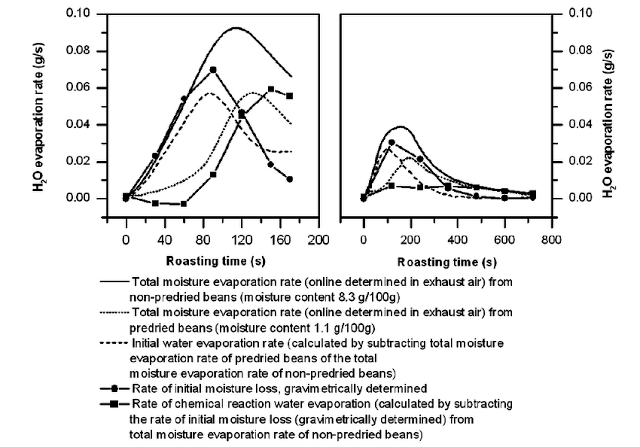
Remember this picture?
In the picture on the left, high temperature and fast drying will retain more water, while in the picture on the right, low temperature and slow drying, then after half the process of about 360 seconds, the evapotranspiration ratio has reached the lower limit, indicating that the water content in the Mena stage is already quite low.
In contrast, the meaning of rapid passage through the rubber state does not seem to have a direct relationship with time, but rather with how much water can evaporate during the dehydration stage (before turning yellow).
Suppose Tian Lao's argument of chlorogenic acid decomposition and dehydration condensation is correct, do you know how to bake it? For example, if you plan to bake the beans at the end of 12 minutes, then set the firepower and throttle to the extent that you can dehydrate and turn yellow at 5, 30, 6, 00, and then stimulate the fire to increase the fragrance or adjust the firepower down to master the development of various fragrances. This is another story.
Then think again, if you want to bring more water into the Mena stage, how to bake it?
Important Notice :
前街咖啡 FrontStreet Coffee has moved to new addredd:
FrontStreet Coffee Address: 315,Donghua East Road,GuangZhou
Tel:020 38364473
- Prev

How exactly is raisin honey treated? Orange honey treatment? CO2 yeast?
Professional barista communication, please pay attention to coffee workshop (Weixin Official Accounts cafe_style) coffee fruit raisin honey treatment exactly how to deal with? At present, I understand the process flow is [double fermentation honey treatment], that is, first the coffee fruit is dried into raisins, then the peel honey treatment fermentation, the fermentation flavor will be more intense, and pectin preservation than other
- Next

Coffee shop music recommendation, coffee time with coffee music is the most suitable! Come and hear it.
Professional barista communication please follow the coffee workshop (Wechat official account cafe_style) do you also like to go to the coffee shop for coffee? What attracts you to the coffee shop for coffee? Dessert? Environment? atmosphere? Or can people precipitate the soul of the music? This time we are going to share with you some good coffee music recommendations and want to have a cup of incense when you are alone.
Related
- Beginners will see the "Coffee pull flower" guide!
- What is the difference between ice blog purified milk and ordinary milk coffee?
- Why is the Philippines the largest producer of crops in Liberia?
- For coffee extraction, should the fine powder be retained?
- How does extracted espresso fill pressed powder? How much strength does it take to press the powder?
- How to make jasmine cold extract coffee? Is the jasmine + latte good?
- Will this little toy really make the coffee taste better? How does Lily Drip affect coffee extraction?
- Will the action of slapping the filter cup also affect coffee extraction?
- What's the difference between powder-to-water ratio and powder-to-liquid ratio?
- What is the Ethiopian local species? What does it have to do with Heirloom native species?

