The physical Theory of Milk Coffee pattern
It plays an important role in barista work. Milk bubbles are two of the situations of particular concern. Before going into the detailed introduction of these two kinds of milk bubbles, let's take a look at some basic theories related to milk bubbles.
So, how does milk produce milk foam?
[1] the direct reason is that some bubbles are brought into the liquid, and in milk bubbles, these bubbles are made more stable by a layer of inclusions around them (by increasing surface tension). In most edible milk foams, protein acts as this inclusion, because part of the protein molecule is hydrophobic and repelled by water, so this part of the protein molecule is also more likely to come into contact with substances other than water. In general, protein molecules are rolled up in aqueous solution, and their hydrophobic parts are inside the molecules; for them to form milk bubbles require some operations, such as heating (or, in the case of egg whites, strong beating). The bubble acts as a moderator of the hydrophobic part of the protein molecule, while the protein molecules are arranged linearly at the gas-water intersection-that is, the surfactant.
[2] Why did the milk foam burst? If it is a milk bubble formed by surfactant in water, then the main cause of rupture is water loss.
The bubbles pull each other by gravitation, and the surfactant is slowly consumed. As a result, the foam becomes fragile and inelastic, so that it finally breaks. However, this does not answer why mistakes may occur when using steam to beat milk foam, and in this case, the main cause of foam rupture is fat. Fat and water are immiscible because water has polarity and fat has no polarity.
[3] part of the protein is also rejected by water because of its lack of polarity. If fat is also present in the milk bubble system, then there is another option for the non-polar part of the protein-it can wrap small "bubbles" of fat. That's why fat makes the foam unstable, and that's why your steam milking is over. (by the same token, if you want to dispose of egg whites, never mix egg yolks in them.)
[4] it is not perfect, but it vividly explains the reason for the combination of surfactants and bubbles.
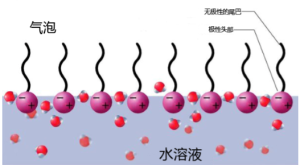
In milk foaming, the role of surfactant is played by a whey protein called β-lactoglobulin. (this is not necessarily the only protein that plays a role in the foaming process, but it is the most important one.) the reason for many milk not foaming is the decomposition of milk fat, and free glycerol from triglycerides hinders the foaming process. For barista, the role of water loss here is slightly different. The process of water loss is related to viscosity. The thicker the liquid is, the slower the water loss is. Therefore, in general, whole milk foams more easily. Compared with skimmed milk, the foam has long-lasting moisture. It will not make the next coffee flower more difficult because it dries quickly. You can use superb technology to make better and more stable foam. (only before the milk temperature reaches the final temperature.) this is because you have made a stable foam before the milk fat reaches the body temperature, at this temperature, the cream will turn into oil, and the oil will destroy the foaming process.
Important Notice :
前街咖啡 FrontStreet Coffee has moved to new addredd:
FrontStreet Coffee Address: 315,Donghua East Road,GuangZhou
Tel:020 38364473
- Prev

Common sense of espresso Crema analysis of oil in Italian coffee machine
Crema (grease) I don't understand why we make Crema so much more complicated than itself. Crema is often seriously misdescribed, the most common of which is an emulsified oil. Well, I think it's fair to say that to a very small extent. As far as we know, the amount of oil obtained by Espresso in the extraction process is unique. In the process of Espresso extraction, the content of steam
- Next

Coffee common sense introduction to coffee taste of various coffee producing countries in Africa
BB Manor Coffee in Kilimanjaro, Tanzania. Bourbon: African countries: Tanzania: BB Manor flavor: blackcurrant or lime sweet and honey sweet, rich peach, plum and drupe cocoa aromas that linger long after such sweet drinks, and the overall sense of balance is also great. Rwanda washed Arabica coffee Rw.
Related
- Beginners will see the "Coffee pull flower" guide!
- What is the difference between ice blog purified milk and ordinary milk coffee?
- Why is the Philippines the largest producer of crops in Liberia?
- For coffee extraction, should the fine powder be retained?
- How does extracted espresso fill pressed powder? How much strength does it take to press the powder?
- How to make jasmine cold extract coffee? Is the jasmine + latte good?
- Will this little toy really make the coffee taste better? How does Lily Drip affect coffee extraction?
- Will the action of slapping the filter cup also affect coffee extraction?
- What's the difference between powder-to-water ratio and powder-to-liquid ratio?
- What is the Ethiopian local species? What does it have to do with Heirloom native species?