Coffee extraction rate, coffee soluble matter, extraction rate and TDS complete
Once the water enters the coffee cell, it begins to dissolve the soluble substances in it. After being fully dissolved, the solution formed by water and these soluble substances is known as coffee, but not all soluble substances are good.
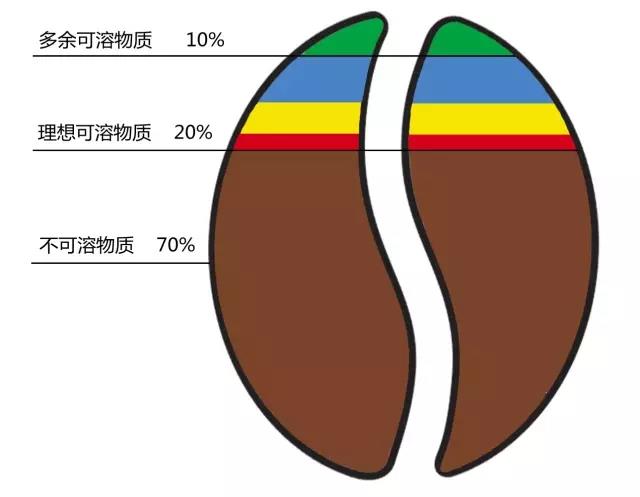
Of the total weight of coffee beans, only 30% are water-soluble, and the remaining 70% are water-insoluble fibers and carbohydrates.
Of these 30%, only 20% are good soluble substances ("ideal soluble substances"), and the remaining 10% are bad soluble substances ("excess soluble substances"), which make coffee taste bad. In order to make coffee more delicious, we should extract as many "ideal soluble substances" from coffee as possible, and avoid extracting "excess soluble substances" as far as possible.
The good news is that "excess soluble substances" usually dissolve slowly, so controlling brewing time is crucial to the quality of coffee.
The longer the contact time between water and cells, the more soluble substances extracted.
In the coffee industry, "extraction rate" is used to describe the ratio between the total amount of soluble matter locked in coffee beans and the total amount of soluble matter extracted.
In principle, the longer the water comes into contact with coffee cells, the more soluble substances will be extracted.
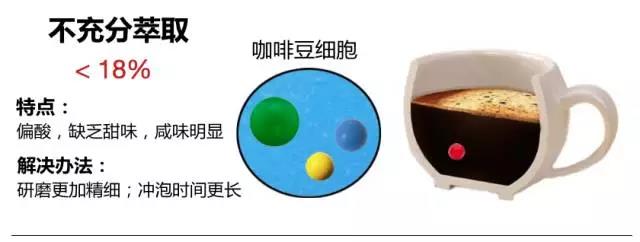
The American Fine Coffee Association (SCAA) officially gave an ideal extraction rate of 18-22%, which means that the total weight of soluble substances dissolved in water should be 18-22% of the original weight of coffee beans. If the value is lower or higher than this value, the taste of coffee will not be ideal, as shown in the following figure:
Water will only extract the soluble substances that come into contact with.
After understanding the coffee extraction process from a microscopic point of view, let's look at the role of water in the coffee extraction process from a macro point of view. For the sake of explanation, let's think of the coffee bean as a two-dimensional plane (in fact, the coffee bean is a three-dimensional solid, as pictured above).
The picture above shows a coffee bean soaked in water. Water can only touch the surface cells of coffee beans, that is, the parts marked in bright blue.
Our goal is to get water into full contact with all the cells of the coffee bean, so we have to let the water into the inside of the coffee bean.
Grind coffee to expose water to more coffee cells
By grinding coffee beans into smaller particles, we can expose water to more coffee cells. The smaller the particles, the more coffee cells the water comes into contact with.
The longer the time, the deeper the immersion of water into a single particle.
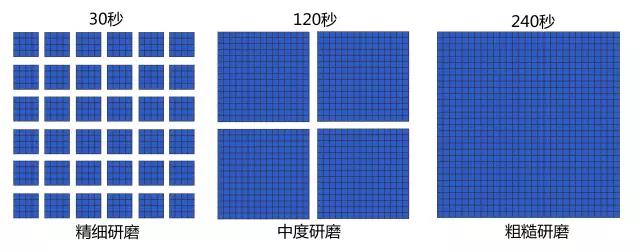
Think of a single coffee powder particle as a box with 90 cells (30x30, pictured above). In the leftmost box, the contact time between the water and the cell is 30 seconds, during which time the water is immersed into only the two layers of cells located in the outermost part of the box.
When the time reaches 120 seconds, the water has been immersed into the 15th layer. If you stop brewing at this moment, the central cell still retains its original state, and the soluble substance in it is still locked in the cell, which should be considered "inadequate extraction".
When the time reaches 240 seconds, all the water has been immersed in all the cells and all the soluble substances have been fully extracted.
The particle size determines the extraction rate.
What happens if you change the degree of grinding (that is, the particle size of a single coffee powder)? First of all, let's assume that the brewing time and the total number of cells of coffee powder with different degrees of grinding are the same (pictured above).
Due to the difference in particle size, it is possible for us to fully extract all the soluble substances from the coffee in 30 seconds.
Particle size does not determine the type of substance to be extracted, but it can determine the time it takes for water to be immersed in all coffee cells.
Now, do you understand the effect of brewing time and grinding degree on coffee extraction? The two complement each other, and the growth and decline of each other.
Extraction is to find a balance between time and grinding degree.
What happens if we use the same time (for example, 120 seconds) to extract coffee powder with different grinding degrees?
As we have just said, if water is in contact with coffee for too long, it is possible to over-extract and release some "excess soluble substances". As shown in the leftmost box above, the cells shown in orange are those "excess soluble substances".
In the middle box, we successfully extracted all the "ideal soluble substances", that is, 20% of the total weight of coffee. At this time, the time and the degree of grinding reached a perfect balance, all the "ideal soluble substances" were fully extracted, and all the "excess soluble substances" were still locked in the cell.
In the box on the right, due to insufficient contact time between the water and the coffee powder, the water failed to extract all the "ideal soluble substances", and some of the substances were still locked in the cells.
From the above picture, we can draw another conclusion, that is, the uniformity of the particle size of coffee powder has a great influence on the extraction degree of coffee. If the size of coffee powder particles is uneven, it will result in excessive extraction of some particles while others are still inadequate.
Concentration is the ratio of soluble matter to water.
After understanding how to completely extract all the "ideal soluble substances", let's talk about the "concentration", that is, the soluble matter ratio (TDS).
TDS refers to the ratio of soluble matter to water in a cup of coffee. Due to individual differences and taste preferences, everyone has a different understanding of TDS. Generally speaking, 1.15% Mel 1.35% is the ideal ratio of soluble matter.
In other words, one part of soluble substance needs to be mixed with 99 parts of water for the coffee to taste good.
The soluble substances extracted determine the taste of coffee, and TDS can show the intensity of these flavors. If taste is compared to "music", TDS is like "volume".
With the increase of TDS, the taste of coffee will become stronger, but if the concentration of some flavors is too high, it will outweigh other more subtle and delicate flavors. The following figure shows the taste characteristics of coffee under different TDS:
Extraction rate and soluble matter ratio (TDS) are the two key factors to measure the quality of coffee. I hope that the content of this article can give you a preliminary understanding of the process of coffee extraction, as well as the concept and meaning of extraction rate and TDS.
Important Notice :
前街咖啡 FrontStreet Coffee has moved to new addredd:
FrontStreet Coffee Address: 315,Donghua East Road,GuangZhou
Tel:020 38364473
- Prev

Professional coffee roasting | how should Rosa Geisha coffee be roasted?
Professional barista communication Please follow the coffee workshop (Wechat official account cafe_style) I think even people with loose pockets will not use Geisha as a practice bean, a pot of practice, maybe so, when drinking Geisha, the mind will inevitably fly over a lot of unknown totems. Recently, there is a 90 + Geisha Super Red, which is bidding for US $5000 a kilogram. People who have drunk it are full of praise.
- Next
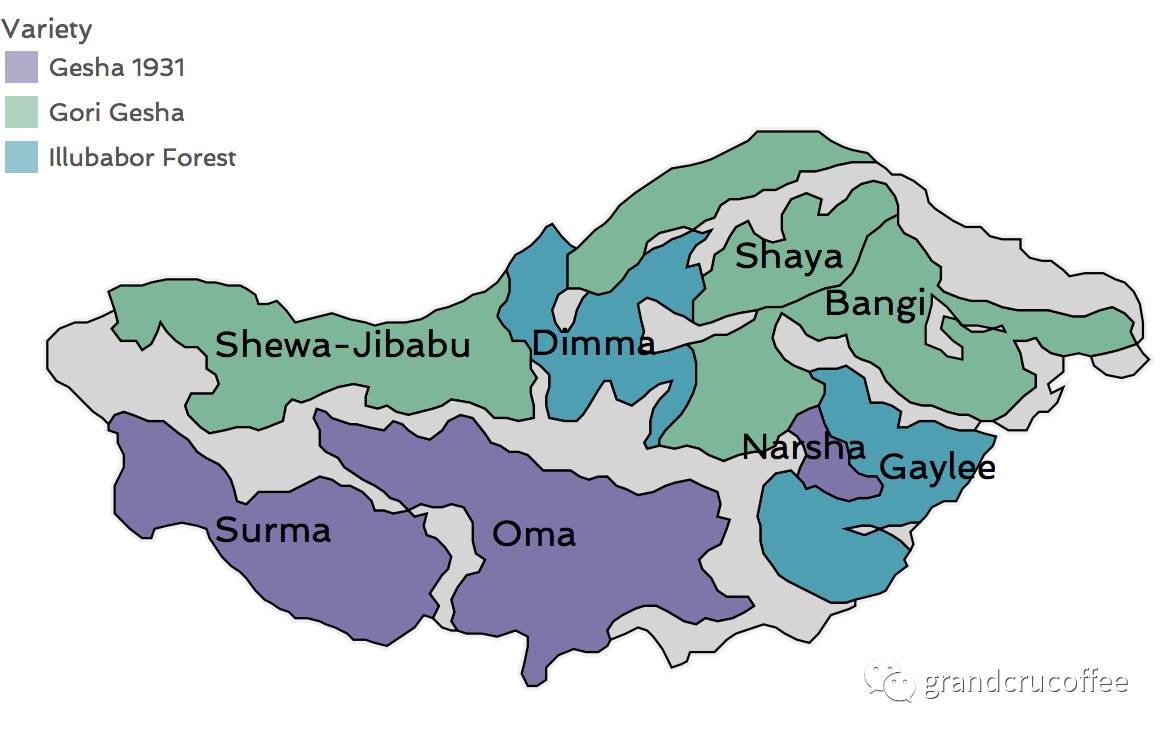
About the background of Rose Xia Village Coffee Manor
What is the difference between going to Dixixia Village and an ordinary Ethiopian manor? What do OMA and SURMA refer to in the batch number? What does the series of numbers 51, 72 and 59 mean? What's the difference between Gesha1931 and Gori Gesha? Next, the editor will give you answers one by one. Manor background Adam (Adam Overton) and Rachel (Samuel Rachel) have been around since 2011.
Related
- Beginners will see the "Coffee pull flower" guide!
- What is the difference between ice blog purified milk and ordinary milk coffee?
- Why is the Philippines the largest producer of crops in Liberia?
- For coffee extraction, should the fine powder be retained?
- How does extracted espresso fill pressed powder? How much strength does it take to press the powder?
- How to make jasmine cold extract coffee? Is the jasmine + latte good?
- Will this little toy really make the coffee taste better? How does Lily Drip affect coffee extraction?
- Will the action of slapping the filter cup also affect coffee extraction?
- What's the difference between powder-to-water ratio and powder-to-liquid ratio?
- What is the Ethiopian local species? What does it have to do with Heirloom native species?

