Chemical reaction and temperature during coffee roasting changes of reactive substances in coffee roasting process

Professional coffee knowledge exchange more coffee bean information please follow the coffee workshop (Wechat official account cafe_style)
Professional coffee roasting | is coffee roasting too complicated? A picture teaches you about the baking process.
Recently, I have been looking at the reaction of chemical components in baking.
Sucrose: melting point 187.8 ℃-> sugar? Complex = > caramelization-> water CO2 escapes to produce an explosive First Crack phenomenon. I measured the temperature of the beans in the drum of the baking machine 190 MULFUR 196 ℃.
Cellulose (lignin): coffee cell wall component, resulting in disintegration of cell wall damage at 230℃-> second explosion Second Crack phenomenon I measured bean temperature 211 / 216 ℃ in the drum bean of the roaster.
Trigonelline (Trigonelline): pure crystal 217.9 ℃ begins to degenerate, when bean temperature 192.2 ℃ begins to degrade, when bean temperature 229.4 ℃-> 85% degrades, trigonelline degradation degree (rate) is the key index to determine the best baking reaction ratio.
Therefore, the loss rate (degradation rate) of trigonelline after baking is 50% Mur80%, which is decomposed into a variety of compounds, including non-volatile nicotinic acid and 29 volatile substances, of which 9 are coffee aromatic substances.
Nicotinic acid: coffee raw bean nicotinic acid often exists in cellulose. Nicotinic acid derives from soluble substances during baking. Nicotinic acid derivatives show good acidity and clean aftertaste (clean finish) in coffee, so nicotinic acid derivative rate is another index to determine the best reaction ratio of roasting.
Ambient temperature (ET): a specific chemical reaction of roasted coffee has a temperature range that produces a good flavor. This temperature range is the ambient temperature.
System energy (BTU): the baking process provides the energy BTU gradient (time-heating), which determines the chemical reaction change rate, which is related to the system energy transfer efficiency (STE). There is an optimal reaction rate (BRR), which provides better performance in the coffee cup.
The optimal reaction rate (BRR): there is a linear relationship between the degradation rate of trigonelline and the derivative rate of nicotinic acid when it is concentrated in the ambient temperature (ET). When the baking reaction distribution is different under ET/BTU/STE, the ambient temperature (ET) of the optimal reaction rate (BRR) (ET) is 205 ℃ 218 ℃ by default.
Maximum ambient temperature (MET): when the caramelization of sucrose begins not to lose temperature, otherwise there will be thermal hysteresis effect, so it is necessary to ensure the temperature of the baking environment to provide system energy transfer for chemical reactions, and too high will cause the optimal reaction rate (BRR) to be too fast, so MET is generally as high as 271℃.
All right! Is the temperature sensor correct?!
1. In the past, 270 ℃ was measured with a pointer thermometer in the oven baking room, where the radiant heat was the highest.
two。 When the bean temperature was measured in the roaster, the second burst point of 227 ℃ was measured, and the innermost side was near the iron plate, which was also the temperature point measured by the machine.
3. Because of the drum and direct fire type, it is difficult to have a real bean warm point. At present, it is closest to the measuring point of bean temperature, and each is covered with 2--3cm baked beans.
This data should be very close to the bean temperature.
Kenya AA 250g
An explosion of 15, 000, 39, 30, 194 ℃.
A dense 16-year-old, 39-year-old, 30-year-old, 19-7-℃
The end of one explosion is 17 years old 39 percent 30 "204 ℃.
The second explosion is 18 years old. 39 percent. 30 "211 ℃.
At the beginning of the second explosion, 18 days later, 39 ℃ 55 "under the bean Full City.
I do not know whether this temperature measurement is correct or not, and the BRR relationship?
Above, use K-Type temperature measuring rod. There is no money for correction. Do it yourself
1.100 ℃ correction, boil with hot water. This is quite accurate. Other pointers are poor, Mercury tube difference is 3 Murray 5 ℃.
two。 Room temperature 25 ℃ correction, three cold air temperature control 25 ℃, temperature measurement point cold air temperature control point, 24 ℃ compressor start, 26 ℃ compressor
Stop, fortunately, the temperature measurements of the three air-conditioners themselves are quite accurate.
3. Low temperature 0 ℃ correction, refrigerator (freezer compressor stop-18 ℃, fresh-keeping room 0 ℃, lowest cold storage room 4 ℃.
Ice + a small amount of water.. After stabilizing, the water temperature is 1.6 ℃ 2.5 ℃, and the surface of the ice cube is 0 Murray 1.3 min.
4. In addition, the fire temperature of the small gas stove is 1150ml 1280 ℃.
Basically, the k-Type thermometer can still be used, but there should be a gap between the coating temperature of a small amount of roasted coffee beans and the bean temperature. People
Probat L12 showed that the temperature of the first burst bean was 186 ℃ and that of the second burst bean was 225 ℃. There is still a gap between my test and this.
Sweet maria's added logging temperature probes to the Probat Lmuri 12
Forget it! Temperature is not that important to coffee. Coffee is baked by feeling. Coffee depends on the mood! XD
First catch some coffee raw beans and the content of compounds after baking

Chemical structure and changes of Coffee
Coffee can spread all over the world within a hundred years and become a worldwide beverage, which has its own attractive characteristics. Among all the drinks, coffee can be said to be the most artistic, skillful, changeable and controversial food, and the main key to the formation of these characteristics lies in the chemical composition and chemical changes produced during processing. Therefore, the chemical composition of coffee and the chemical changes produced in the process of coffee are the most important basic knowledge for the study and understanding of coffee.
Arabica coffee raw beans are like a warehouse full of chemicals. At present, scientists have identified more than 2, 000 known ingredients, of which 7 to 850 are aromatic ingredients, which can be called the most fragrant in the human diet. There are only 150 kinds of vanilla used for seasoning in the catering industry, and the mellow composition of wine is not nearly as rich as coffee. In addition, it also contains a lot of high-quality acids, including citric acid, malic acid, acetic acid, phosphoric acid and quinic acid, and other organic acids will be born with the disintegration of carbohydrates in the baking process. Up to now, as many as 34 kinds of organic acids have been determined, of which 15 are volatile. In principle, the concentration of high-quality citric acid, malic acid, phosphoric acid and acetic acid is the highest in moderate baking and decreases with heavy baking. Organic acids have a great influence on the flavor of coffee.
1. Moisture: the water content of coffee beans varies greatly with different processing stages and products. The moisture content of wet coffee beans with film is about 50%, while that of dried raw coffee beans is about 10%. The moisture content of roasted coffee beans is only about 5%. The existence of water in coffee beans, like other kinds of food and beverages, contains a considerable amount of hydration colloidal macromolecules, such as proteins and polysaccharides, so that water exists in coffee beans in many different physical and chemical ways.
two。 Minerals: although the content of minerals in coffee beans is small, accounting for about 4% of the dry weight of raw coffee beans, it is very important. It contains many different elements, of which potassium is the most, accounting for about 40% of all minerals, followed by calcium, magnesium, phosphorus, sodium and sulfur. There are many other trace elements at the ppm level, such as zinc, manganese, copper, rubidium and so on. In addition, the most important factors affecting the mineral content and species of raw coffee beans are soil and fertilization in the process of cultivation. During the preparation of coffee, at least 90% of the minerals can be extracted from roasted coffee beans, so the relationship between the content of potassium or other minerals and the yield of coffee soluble matter can be used to evaluate the extraction rate of coffee.
3. Carbohydrates: carbohydrates in coffee beans can be divided into polysaccharides and low molecular weight sugars, which contain carbohydrates such as monosaccharides, disaccharides and trisaccharides. In addition, it can be divided into reducing sugar and non-reducing sugar. It also contains some derivatives, such as pectin. The contribution of carbohydrates to coffee lies in its taste, aroma and color. In terms of flavor, carbohydrates will not only emit coffee aroma after baking, but also absorb volatile aroma, making coffee show a special flavor. The carbohydrate content of raw coffee beans is about 40%, of which about 8%-10% belong to the caramelization of low molecular sugars in the baking process and are converted into caramel pigments and other components, while polysaccharides and pectin are mainly cellulose or lignin.
a. Low molecular weight sugars: sucrose is the most important free sugar in raw coffee beans, and its content varies according to variety, source and maturity. Other simple sugars, including reducing sugars, can also be detected in the extract of raw coffee beans. Raw coffee beans also contain glucose and fructose. After roasting, the change of low molecular sugar varies according to the degree of roasting, and the loss of sucrose is the fastest. The loss rate of light baking is 97%, moderate baking is 99%, and heavy roasting is 100%. Others, such as glucose, fructose and arabinose, also have considerable losses.
b. Polysaccharides: polysaccharides are very important components in raw coffee beans, accounting for about 40%-50% of the dry matter. According to the type, there are polygalactose, polyicoses, polyarabinose and cellulose, which are the substances that make up the plastids of coffee beans and are related to the hardness of coffee beans. Polysaccharides can still be preserved in a considerable amount after baking. According to the data obtained from a study, there is not much difference between different baking degrees, and the retention rate is between 70% and 75%. The retention rate of cellulose is the highest and that of polyarabinose is the lowest.
c. Pectin and lignin: pectin is a combination of a variety of polysaccharides, its main composition is divided into galactoic acid polymer, rhamnose and so on, its content is more than 3%. Lignin is the insoluble residue left after the plant is treated with sulfuric acid and caustic alkali, that is, the so-called coffee fiber, with a content of about 2.4%.
4. Nitrogenous compounds: the nitrogenous compounds in raw coffee beans can be divided into plant alkaloids, trigonelline (Trigonelline), nicotinic acid, protein and free amino acids, etc., as follows:
a. Plant alkaloids: mainly caffeine (Caffeine), the content of raw coffee beans varies greatly from variety to variety. In the case of arabica coffee raw beans, the average is about 1.2-1.5%. In recent years, low-caffeinated coffee beans have been cultivated in Java and C ô te d'Ivoire, with a caffeine content of only 0.2%. Caffeine can be removed by a variety of processing methods, and the caffeine content of raw beans of processed decaffeinated coffee is less than 0.3%. Caffeine can be said to be the spirit of coffee, but also the most controversial issue. Although caffeine has no odor, it has a remarkable bitter taste. However, it is impossible to judge the amount of caffeine by the bitterness of coffee, because the bitter taste of caffeine accounts for only a small part of the bitter taste of coffee, so there is not much effect on the bitterness of decaffeinated coffee. After being digested by the human body, caffeine can be quickly absorbed and metabolized and excreted from the urine. The most significant physiological effect of caffeine on the human body is the stimulation of the central nervous system. As for the dose required for changes in brain activity, which is much higher than the normal intake, the other physiological effects that attract more attention are the effects on blood pressure, the direction of the heart and blood vessels.
b. Trigonelline (Trigonelline): trigonelline is a colorless crystal with hygroscopicity and excellent solubility in water. It also has a low degree of physiological effects, mainly for the central nervous system, bile secretion and intestinal peristalsis. The direct effect of trigonelline on the quality of coffee is very small, and its bitter taste is only 1/4 of that of caffeine. Trigonelline decomposes quickly during baking, and its loss rate is between 50% and 80%, which varies according to baking temperature and time. In addition, trigonelline decomposes to produce a variety of compounds, including non-volatile nicotinic acid. And 29 kinds of volatile compounds, among which 9 kinds of coffee aroma compounds have been identified.
B1. Nicotinic acid: the content of nicotinic acid in raw coffee beans is very small, but it increases after baking, mainly because it comes from the decomposition of trigonelline. But the results show that nicotinic acid continues to break down into volatile chemicals at high baking temperatures, so the real increase is not much.
5. Protein and amino acids: in terms of crude protein, the content of raw coffee beans is about 13% Mel-16%. If nitrogen compounds such as caffeine and trigonelline are deducted, the real protein content is about 8.8% Mel-9.7%. Raw coffee beans also contain a variety of enzymes, such as lipopolytic enzymes, proteolytic enzymes, carbohydrate hydrolytic enzymes, galactose hydrolytic enzymes and peroxide enzymes. Coffee raw beans contain about 0.15%-0.25% free amino acids, which have a high degree of influence on the flavor of coffee and less effect on the taste. The protein content after baking is about 3.1%-4.0%.
6. Chlorogenic acid (chlorogenic Acidss): after baking, this substance will disappear through different actions, resulting in extremely complex products, which are closely related to the quality of coffee. The content of chlorogenic acid in raw coffee beans is about 8%-10%, that is, hydroxy cinnamate isomers of quinic acid (a common plant ingredient), which is found in most plants and fruits. Chlorogenic acid is the original culprit of acerbity and bitterness of coffee. Fortunately, chlorogenic acid will be destroyed during baking, forming lactone binding to non-acid quinine lactone (quinine compound). During the roasting process of chlorogenic acid in raw coffee beans, about half of the chlorogenic acid will disappear, and the content of chlorogenic acid will be reduced to about 4%-5% after baking.
7. Lipids: the lipids of raw coffee beans are composed of coffee oil (Coffee Oil) in the endosperm and wax in the outer layer of coffee beans. Coffee oil not only contains triglycerides, but also contains equivalent other lipid components, which forms the characteristics of coffee, which is very important for coffee. The content and composition of coffee oil in raw coffee beans vary according to varieties. The average content of raw coffee beans is about 12%-15%. About 97% of the ingredients are still preserved in the form of lipids after baking.
8. Volatile compounds: volatile compounds are the main source of coffee flavor and are especially important for coffee quality. There are many kinds of volatile substances in coffee, and their existence will affect the aroma quality of coffee. Its main source is derived from non-volatile substances in raw beans which are cut off or derived after reaction during the baking process. The aroma and flavor of coffee are formed as a result of thermal decomposition, other reactions or compositions, such as sugars, amino acids, organic acids and phenolic compounds. The factors affecting the volatile components of coffee include the variety of coffee beans, cultivation climate, soil conditions, storage of raw beans, baking temperature and time, baking equipment and so on.
Raw coffee beans do not contain the special aroma of coffee, so they are not eaten directly and must be roasted before a large number of volatile aroma substances are produced. After roasting, the main volatile aroma components of raw coffee beans are more than 660 by analysis, which is the food with the largest variety of volatile aroma components in all foods and beverages. The aromas produced in the baking process, such as hazel, cream and caramel, or with grass, smoking, burning, spice and bitterness, are often volatile substances. In addition, the difference of roasting degree will also affect the flavor characteristics of coffee.
Caramelization is a link that has the greatest impact on the flavor of coffee. After six to seven minutes of baking, raw beans absorb a lot of heat and start the pyrolysis reaction. Some sugars are converted into carbon dioxide, and water continues to evaporate. New aromatic components gradually develop to form the so-called coffee oil, and combine with hundreds of aromatic substances such as nicotinic acid, citric acid, quinic acid, malic acid, acetic acid, caffeine and so on. The pyrolysis reaction can last until the second explosion. Although caramelization is an important process to awaken aromatic elves, with the prolongation of baking time, some ingredients will also be carbonized to form bad astringent substances. How to obtain the highest caramelization and minimize carbonization at the same time seems to be the biggest challenge for bakers.
.
Important Notice :
前街咖啡 FrontStreet Coffee has moved to new addredd:
FrontStreet Coffee Address: 315,Donghua East Road,GuangZhou
Tel:020 38364473
- Prev
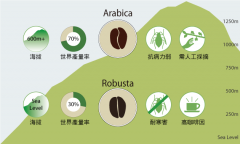
How much is Arabica coffee per jin? Characteristics of Arabica coffee bean, a native tree species of coffee
Professional coffee knowledge exchange more coffee bean information Please note that the coffee workshop (Wechat official account cafe_style) is also a member of Arabica, why are some coffee beans so expensive? There are tens of thousands of coffee tree species in the world, and many varieties are gradually emerging to say hello to everyone. I also hope that while tasting coffee, we can understand where coffee comes from and enter.
- Next

Coffee bean roasting process shows coffee talent teaching coffee roasting experience
Professional coffee knowledge exchange more coffee bean information please follow the coffee workshop (Wechat official account cafe_style) professional coffee roasting | coffee roasting is too complicated? A picture teaches you that good roasting can enhance the local conditions and taste of coffee beans. If the roasting is too bitter or not ripe, the coffee will lose its taste. Have rich baking experience
Related
- Beginners will see the "Coffee pull flower" guide!
- What is the difference between ice blog purified milk and ordinary milk coffee?
- Why is the Philippines the largest producer of crops in Liberia?
- For coffee extraction, should the fine powder be retained?
- How does extracted espresso fill pressed powder? How much strength does it take to press the powder?
- How to make jasmine cold extract coffee? Is the jasmine + latte good?
- Will this little toy really make the coffee taste better? How does Lily Drip affect coffee extraction?
- Will the action of slapping the filter cup also affect coffee extraction?
- What's the difference between powder-to-water ratio and powder-to-liquid ratio?
- What is the Ethiopian local species? What does it have to do with Heirloom native species?

