Introduction to coffee roasting training-basic chemical reactions in the coffee roasting process

Professional coffee knowledge exchange more coffee bean information please follow the coffee workshop (Wechat official account cafe_style)
A complete collection of coffee roasting techniques | the purpose of coffee roasting and the characteristics of coffee beans
The word MET (maximum ambient temperature) is rarely seen except in home-barista.com forums. MET is not a number, but a curve connected by the highest ambient temperature of each time segment during the baking process, such as the highest curve in the following figure.
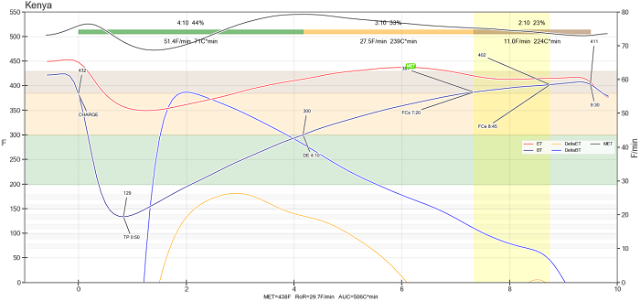
Because of this Quest Me driving the roast with MET of Quest M3 MET, I found a good article. Although it is very difficult for me, I try my best to summarize it.
Many thermal and chemical reactions occur in the baking process, such as decarboxylation, dehydration of quinic acid groups, fractionation, polymerization, isomerization and complex reactions of sugars. The protagonists of these reactions are monosaccharides, sucrose, chlorogenic acid, free amino acids and trigonelline. During the reaction, the monosaccharides in carbohydrates are polymerized and degraded, and 30% of the polysaccharides are decomposed according to the degree of baking.
Sucrose: disaccharide consisting of d-glucosyl and d-fructose groups
Sucrose is the main sugar in coffee beans. The melting point of sucrose crystallization is about 320murmur199C, but sucrose can be degraded into glycosides and water since 194F (90C). Then caramelization starts at 338Muth392F (170Muth200C), which produces water vapor and carbon dioxide, causing the beans to burst (burst). From about 356F (180C), the chemical reaction of beans enters an exothermic state. At the beginning of the caramelization reaction, the substance of coffee does not exothermate. one explanation is that the substance involved in the exothermic reaction interrupts the long-chain polymerization and cross-links to the composition of other components. The true melting point, caramelization and subsequent conversion of sucrose are affected by the presence of moisture, ammonia and protein. The degree of caramelization of deep-roasted coffee will be higher, so it is an ideal method to distinguish the degree of roasting by the degree of caramelization.
Cellulose: a long linear polymer of anhydrous glucose
Cellulose is the main component of the cell wall, some of which are in an orderly crystalline state and some are disordered non-crystalline. Non-crystalline cellulose is easier to react to heat, while fiber crystallization may not be affected by heat at all. After baking, the natural cellulose (cellulose I) will be converted into polycrystalline cellulose III and fiber IV. The structural transformation of cellulose contributes to the uniform heat conduction of the baking process and the homogenization of the bean body. Lignocellulose (a kind of non-crystalline hemicellulose and lignin fiber structure) forms the structure of cell wall and is a high degree of aromatic polymerization. when the temperature dispersed in the bean reaches 446F (230C) or the surface temperature of the bean reaches 536F (280C), the cell wall will be seriously damaged. The second explosion occurred during deep baking is the fracture of the cell wall structure of the bean body, which may be related to the escape of gases produced by lignin and other aromatic substances. It is recommended that the ambient temperature of roasting be controlled below 356F (280C), and if it is safer, the maximum ambient temperature (MET) should be controlled below 520F (271C) to ensure that the beans will not be damaged, enrich the flavor of the coffee, increase the yield, and prolong the shelf life.
Trigonelline: a nitrogenous base of coffee.
Trigonelline is completely soluble in water, so all trigonelline in coffee will eventually be extracted into the cup. Trigonelline is probably the most important cause of excessive bitterness of coffee. 85% of trigonelline will be degraded when the bean temperature reaches 445F (229C), and the beans that reach this temperature are medium-to-deep roasted, which means that the lightly roasted coffee will contain more trigonelline (bitter) and have a lower degree of caramelization. Sucrose is sweeter than caramel, if baked properly, the sweetness of sucrose and the bitterness of trigonelline will cleverly set off each other to form a good taste. The melting point of trigonelline crystallization is about 424F (218C), but the initial degradation temperature is only 378F (192C). The degradation of trigonelline is one of the main indexes to determine the best reaction rate (the best reaction ratio: BRR).
Quinic acid: a member of the carboxyl group
The crystallization melting point of quinic acid is 325F (163C), which is much lower than the general baking ambient temperature. quinic acid is completely soluble in water and has a bit of acidity and sharpness, which adds complex flavor to the coffee cup. Surprisingly, it can also increase the cleanliness of the taste when measuring the cup, and it is a relatively stable substance in the baking process.
Nicotinic acid: a member of the carboxyl group
The melting point of nicotinic acid crystal is 457F (236C), which is usually accompanied by polysaccharide cellulose and becomes soluble in water after coffee roasting. Regardless of the roasting degree of coffee, the proportion of nicotinic acid is proportional to the quality of coffee. Because it can be completely extracted into the cup, it helps the coffee to show bright acidity. Its formation rate is an important control flag for determining the optimum reaction reaction temperature (best reaction ratio temperature) and chemical propagation rate (chemistry propagation rate). In addition, the interaction between melted nicotinic acid and other components significantly increased the intensity of deep flavor.
Ambient temperature (Environment Temperature)
The ambient temperature of baking determines the occurrence of specific chemical reactions. A specific ambient temperature range (temperature window) is very favorable for cup flavor testing, while deviating from this range has a bad effect. But even if it falls in this range, different temperatures will determine the characteristics of various flavors, allowing the baker to strengthen or wear off certain flavor traits to create a personal style. System energy: at any ambient temperature, energy (BTU) and the transfer efficiency of the baking system determine the rate of a particular chemical reaction. A higher energy transfer leads to a faster response. There is a reaction rate interval (window of reaction rates), which can optimize the quality of the cup test, which is called the best reaction rate (BRR: the Best Reaction Ratio).
The best reaction rate (BRR: the Best Reaction Ratio)
When the linear relationship between the degradation of trigonelline and the derivative ratio of nicotinic acid is maintained, the best cup quality will be produced, and the control model of this ratio is the relationship among time, temperature and energy. The ambient temperature (ET) establishes the high temperature pyrolysis space needed for the desired chemical reaction, while the energy value (BTU) and system transfer efficiency (STE) determine the linear relationship between the reaction transmission rate and the degradation of nicotinic acid derivatives to trigonelline. Because the density of coffee beans varies greatly, the distribution of the reaction will be different under any ET / BTU / STE formula, so it takes longer to make the high-density beans uniform. The measurement of bean temperature is a good way to monitor the distribution of baking reaction. The ideal ambient temperature (ET) of the optimal reaction ratio (BRR) is 401m / m 424F (205m / m 218C). Usually 405F (208C) is taken as the predetermined temperature, and the required BTU depends on the heat transfer efficiency of the system.
Maximum ambient temperature (MET: Maximum Environment Temperature)
It is a balanced act to establish a heating environment temperature specification for ideal baking. Although we hope to maintain the BRR temperature and energy level until the target reaction occurs, the BRR temperature is actually much higher than the caramelization temperature of sucrose, and since many baking systems only use simple temperature regulators, care must be taken not to let coffee beans accidentally enter the exothermic reaction too quickly. In addition, it is also important to limit MET, as mentioned earlier, it is important to maintain the structural integrity of cellulose, and lower temperatures will reduce surface evaporation and reduce capillarity (the role of attracting bean components to the surface). By limiting the maximum temperature, the structural loss of cellulose is minimized and the essence of coffee is preserved, so the MET should not exceed 520F (271C). The number of MET and the baking degree of beans are related to the temperature of the beans.
Basic and advanced sharing of coffee roasting experience and knowledge
Important Notice :
前街咖啡 FrontStreet Coffee has moved to new addredd:
FrontStreet Coffee Address: 315,Donghua East Road,GuangZhou
Tel:020 38364473
- Prev

[sharing the experience of hand-brewing coffee] four tips for roasting beans in the middle and deep of hand-brewing
Professional coffee knowledge exchange more coffee bean information Please follow the coffee workshop (Wechat official account cafe_style) encountered in the deep-roasted beans, many people will have this problem when brewing: the taste of coffee is too bitter. Today, I would like to share a little experience so that you can reduce mistakes when baking beans in deep cooking, and keep the taste of coffee at an acceptable level.
- Next

[professional coffee roasting] effect of barrel speed on roasting temperature and time of coffee beans
Professional coffee knowledge exchange more coffee bean information please follow the coffee workshop (Wechat official account cafe_style) Coffee roasting skills Collection | the purpose of coffee roasting and the characteristics of coffee beans about rolling bucket speed, very interesting discussion, especially there are two masters said just the opposite https://www.home-barista.com/home-roasting/drum-speed-t4165
Related
- Beginners will see the "Coffee pull flower" guide!
- What is the difference between ice blog purified milk and ordinary milk coffee?
- Why is the Philippines the largest producer of crops in Liberia?
- For coffee extraction, should the fine powder be retained?
- How does extracted espresso fill pressed powder? How much strength does it take to press the powder?
- How to make jasmine cold extract coffee? Is the jasmine + latte good?
- Will this little toy really make the coffee taste better? How does Lily Drip affect coffee extraction?
- Will the action of slapping the filter cup also affect coffee extraction?
- What's the difference between powder-to-water ratio and powder-to-liquid ratio?
- What is the Ethiopian local species? What does it have to do with Heirloom native species?

