Professional coffee roasting | Mena reaction and caramelization, flavor and hierarchy

Professional coffee knowledge exchange more coffee bean information please follow the coffee workshop (Wechat official account cafe_style)
Professional coffee roasting | is coffee roasting too complicated? A picture teaches you about the baking process.
In the process of roasting coffee, bean bakers always wonder, at what stage does the source of these flavors and layers come into being? What are the control points that lead to it?
As far as I am concerned, the main direction of thinking in the baking process starts with a main goal: what is the use of roasted coffee beans?
Cup test?
Taste?
Comprehensive formula?
A single item?
……
First have a clear goal, and then think about how to achieve it.
And how to make the baking plan in the middle process, and adjust the baking method in the planning process according to the characteristics of the bean baking machine.
Someone asked me how the aroma and layers should be baked, because in the deeper condition, the aroma is obvious, but the level is relatively poor. Is this the condition of conducting heat to eat more?
As far as the formulation of the baking plan is concerned, the more inked part is the stage of entering beans to 150 degrees, because in my personal opinion, heat absorption at this stage is the main work, and of course, the process of heat absorption will also include the generation of various phenomena. and the emergence of these phenomena will cause beans to enter a state of explosion.
To put it simply, it is Mena's reaction to this process!
There are several elements required for the Mena response:
Protein, reducing sugar, high temperature, dry anhydrous.
What is the temperature at which Mena browns? It's about 118 degrees Celsius.
We should also discuss the caramelization reaction: the process of molecular disintegration when sugar is heated.
Sucrose dissolves into a transparent and colorless liquid when it reaches 185 degrees Celsius.
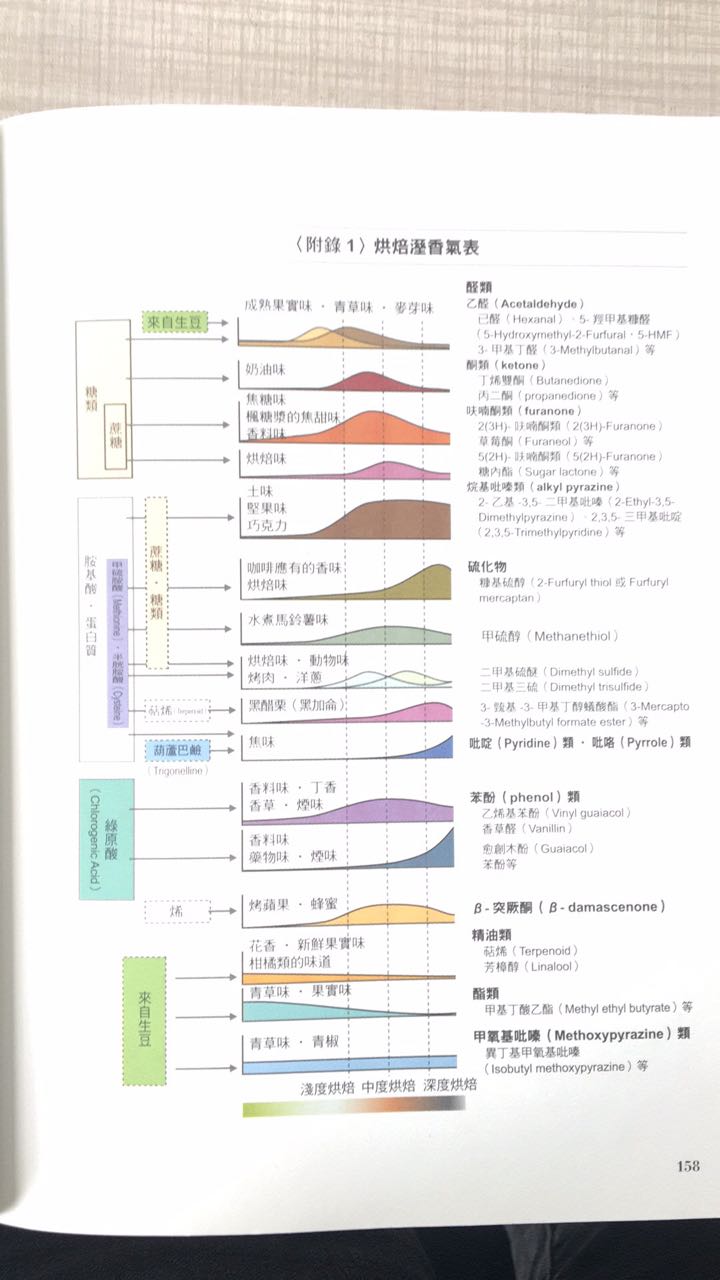
According to the book Food and Cooking: milk, Egg, Meat, Fish, caramelization and Mena reactions each produce the following aromas:
Caramelization: sweet, sour, bitter, fruity, sherry-like smell, cream sugar, caramel, nut-Mena reaction: salty, floral, onion and meat, green vegetables, chocolate, potato and earth, the above caramelized aroma
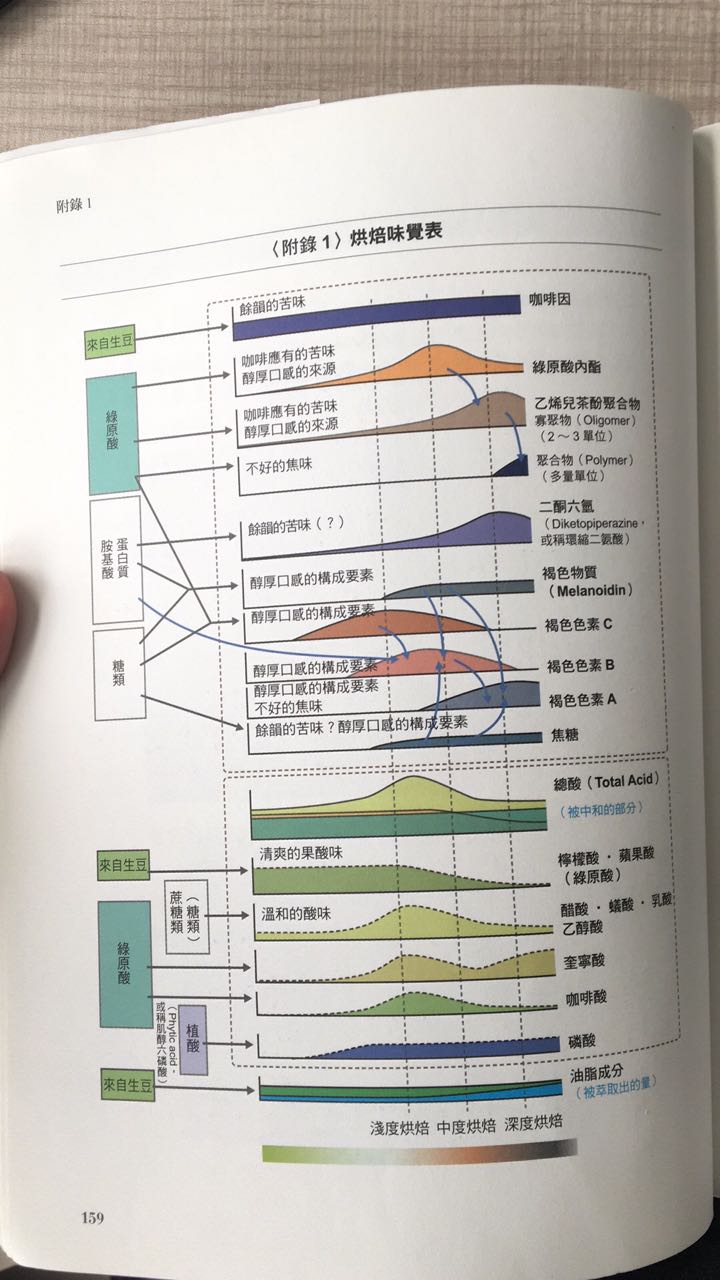
What have you found out from the above points? The above data are mainly for food cooking, but there are the same elements for coffee bean roasting.
Water will not evaporate until it reaches at least 100C, and it is obvious that there is a significant release of water vapor at 130C during the drying process. Has the Mena reaction begun at this stage?
It depends on whether the heat absorption in the front paragraph is enough. Because the surface of the raw bean is different from the bean core in the degree of heat absorption, the fact that the surface has entered the stage of water evaporation does not mean that the bean core also enters this stage.
During the baking process, these reactions are carried out step by step, so it is impossible to directly determine the beginning or end of the reaction at a temperature point. I will personally adjust the start and speed of the Mena reaction from 150 degrees. One point to consider at this time is moisture. One of the elements of the Mena reaction is dry and anhydrous, but coffee roasting is accompanied by a lot of moisture, assuming 100C water begins to be released when heated. Nor does it mean that the temperature measured during the roasting of coffee beans has really reached 100 degrees C. This point can be thought about.
Therefore, when the bean temperature shows 100 degrees C during baking, there will be obvious moisture production only when the temperature reaches 120 degrees C, 130 degrees C. it is necessary to take a reference attitude towards the process of temperature display.
After all, there are errors between practical phenomena and theories.
However, there is no doubt that the coffee still contains water after it has been roasted. Is the Mena reaction process really complete since the water is preserved?
We are looking for the delicious molecules produced by baking, which are all reacted by the substances in coffee beans when heated, and one of the key points of the Mena reaction is that it is dry and anhydrous, and the performance of the baking process, does the release of water produce Mena reaction at the same time?
I think it's different in degree.
Because at the same time the water is released at the same stage (120m / m2 / 130C), it can be considered that the heat absorption of raw beans reaches the temperature at which the water is released, and the temperature of Mena is 18 degrees higher than that of water evaporation. That can explain that the yellowing stage of beans at 150C is the beginning of Mena reaction?
Maybe you can think so?
It may be inferred from the above nonsense that the goal of a high hot air ratio is to allow moisture to move forward in the Mena reaction and to lower the temperature of the Mena reaction, which can prolong the time of the Mena reaction. If the Mena reaction is delayed due to the presence of water, and the caramelization phenomenon gradually occurs, can it be considered that the lack of Mena reaction before the explosion leads to the decrease of flavor? at the same time, the phenomenon of caramelization also enhanced the obvious flavor of caramelization.
That's a big break!
To put it another way: too much water in an explosion will enhance the taste and may also produce a bitter taste.
Another idea derived is that there is less moisture in the first explosion, the Mena reaction is more complete, the aroma is obvious, while the caramelization reaction is less, the lower bean temperature is lower (shallow baking), and the flavor can be more complex.
Can you think so?
.
Important Notice :
前街咖啡 FrontStreet Coffee has moved to new addredd:
FrontStreet Coffee Address: 315,Donghua East Road,GuangZhou
Tel:020 38364473
- Prev

A coffee tree is not a tree? The cultivation method of Coffee and the Standard of fertilization in Coffee Garden
Professional coffee knowledge exchange more information about coffee beans Please follow the coffee workshop (Wechat official account cafe_style) what are the fruits and seeds of coffee? The good quality and type of coffee depends on the cultivation method of coffee the elements of coffee cultivation (the conditions of coffee cultivation) Coffee tree is called a tree, but it is actually an evergreen shrub. If
- Next

For novice coffee lovers, how to find the right coffee beans as soon as possible?
Professional coffee knowledge exchange more coffee bean information please follow the coffee workshop (Wechat official account cafe_style) whether you are new to the pit, or resolutely switch from instant coffee to freshly ground coffee, if you do not know how to find the right coffee beans, the best way is to go to your favorite cafe and ask the local barista or baker.
Related
- Beginners will see the "Coffee pull flower" guide!
- What is the difference between ice blog purified milk and ordinary milk coffee?
- Why is the Philippines the largest producer of crops in Liberia?
- For coffee extraction, should the fine powder be retained?
- How does extracted espresso fill pressed powder? How much strength does it take to press the powder?
- How to make jasmine cold extract coffee? Is the jasmine + latte good?
- Will this little toy really make the coffee taste better? How does Lily Drip affect coffee extraction?
- Will the action of slapping the filter cup also affect coffee extraction?
- What's the difference between powder-to-water ratio and powder-to-liquid ratio?
- What is the Ethiopian local species? What does it have to do with Heirloom native species?

