What chemical reaction changes will take place during the roasting process of coffee beans? What happened to the roasting of coffee beans

Professional coffee knowledge exchange more coffee bean information please follow the coffee workshop (Wechat official account cafe_style)
What chemical reaction changes will take place during the roasting process of coffee beans? What happened to the roasting of coffee beans?
Sucrose: melting point 187.8 ℃-> sugar? Complex = > caramelization-> water CO2 escapes to produce an explosive First Crack phenomenon. I measured the temperature of the beans in the drum of the baking machine 190 MULFUR 196 ℃.
Cellulose (lignin): coffee cell wall component, resulting in disintegration of cell wall damage at 230℃-> second explosion Second Crack phenomenon I measured bean temperature 211 / 216 ℃ in the drum bean of the roaster.
Trigonelline (Trigonelline): pure crystal 217.9 ℃ begins to degenerate, when bean temperature 192.2 ℃ begins to degrade, when bean temperature 229.4 ℃-> 85% degrades, trigonelline degradation degree (rate) is the key index to determine the best baking reaction ratio.
Therefore, the loss rate (degradation rate) of trigonelline after baking is 50% Mur80%, which is decomposed into a variety of compounds, including non-volatile nicotinic acid and 29 volatile substances, of which 9 are coffee aromatic substances.
Nicotinic acid: coffee raw bean nicotinic acid often exists in cellulose, in the baking process nicotinic acid derived from soluble substances, nicotinic acid derivatives in coffee show good flavor acidity and clean aftertaste (clean finish), so nicotinic acid derivative rate is another index to determine the best reaction ratio of baking.
Ambient temperature (ET): a specific chemical reaction of roasted coffee has a temperature range that produces a good flavor, which is the ambient temperature.
System energy (BTU): the baking process provides the energy BTU gradient (time-heating), which determines the chemical reaction change rate, which is related to the system energy transfer efficiency (STE). There is an optimal reaction rate (BRR), which provides better performance in the coffee cup.
The optimal reaction rate (BRR): there is a linear relationship between the degradation rate of trigonelline and the derivative rate of nicotinic acid when it is concentrated in the ambient temperature (ET). When the baking reaction distribution is different under ET/BTU/STE, the ambient temperature (ET) of the optimal reaction rate (BRR) (ET) is 205 ℃ 218 ℃ by default.
Maximum ambient temperature (MET): when sucrose caramelization reaction begins not to lose temperature, otherwise there will be thermal hysteresis effect, so it is necessary to ensure the temperature of the baking environment to provide system energy transfer for chemical reaction, and too high will cause the optimal reaction rate (BRR) to be too fast, so MET is generally as high as 271℃.
Raw beans will change in two stages after baking.
1 physically = > volume, density, volume
2 chemically = > smell, taste, taste
The violent reactions of coffee beans during high temperature baking include Mena reaction (Maillard reaction), caramelization reaction (Caramelization), Stryker degradation reaction (Strecker degradation), and pyrolysis of various sugars, amino acids and lipids. The aroma of coffee is produced by combining the results of these reactions. Different raw beans, different composition, different baking aroma.
Mena reaction (Maillard reaction)
In 1912, Louis Camille Maillard Camille Mena (1878 murmur1936) discovered that the mixture of amino acids and sugars produced yellowish brown substances after heating the aqueous solution. In memory of Louis Camille Mena (Louis Camille Maillard, 1878 murmur1936), we call this reaction the Mena reaction (Maillard reaction).
Non-enzymatic saccharification (nonenzymatic glycation) or Mena reaction (Maillard reaction) is the reaction of reducing sugars and amines in the absence of enzymes to produce yellowish-brown substances, which are collectively referred to as advanced glycation end products (advance glycation endproducts,AGEs).
Mena reaction (Maillard reaction) is a non-enzymatic browning reaction widely distributed in food industry.
This concept is the vision of baking bread. It is better to say that what you see in life is more associative than the theory of chemistry.
It's like the beef is bloody to the delicious entrance... It looks like three / five / seven / well-done
It refers to reducing sugars and amino compounds, such as free. Amino acid, peptides, protein, amine and other compounds react to form glycosaminoglyco. after Amadori or Heyns translocation, they go through a series of dehydration, condensation and polymerization to form dark brown substance (melanoidins) melanin or melanin. In addition to producing melanoidin, hundreds of intermediate molecules with different odors, including reducing ketones, aldehydes and heterocyclic compounds, are produced in the reaction process, which provide delicious flavor and attractive color for food.
Caramelization reaction (Caramelization)
When sugars are no longer in the presence of amino acids, when heated above the melting point, the sugars will dehydrate and degrade, as well as browning, which is called caramelization.
The concept is caramel sauce under the pudding.
Take two sand and add some water to boil it brown. Don't go too deep. Because it will be bitter.
Stryker degradation reaction (Strecker degradation)
The condensation of dicarbonyl compounds with α-amino acids will be accompanied by decarboxylation (CO2), resulting in aldehydes with one less carbon than the original amino acids.
Coffee raw beans, roasted by different people, also have different flavors. Even if it is baked by the same person, the taste will be slightly different each time.
The performance of each roaster is also different. Bakers also use different methods.
What the baker can do is the baking degree.
Every batch of raw beans is every batch of the year. Every time it is processed and preserved because of the climate. The environment is different
Plus the changes in the baking environment... There is no guarantee.
What is guaranteed is to try to taste as much as you want.
Baking coffee is so fun and fun-- that's why I've been specializing in it.
Don't ask for the same flavor as the beans last time.
Every batch of raw beans is every batch of the year. Every time it is processed and preserved because of the climate. The environment is different
Plus the changes in the baking environment... There is no guarantee.
What is guaranteed is to try to taste as much as you want.
Important Notice :
前街咖啡 FrontStreet Coffee has moved to new addredd:
FrontStreet Coffee Address: 315,Donghua East Road,GuangZhou
Tel:020 38364473
- Prev
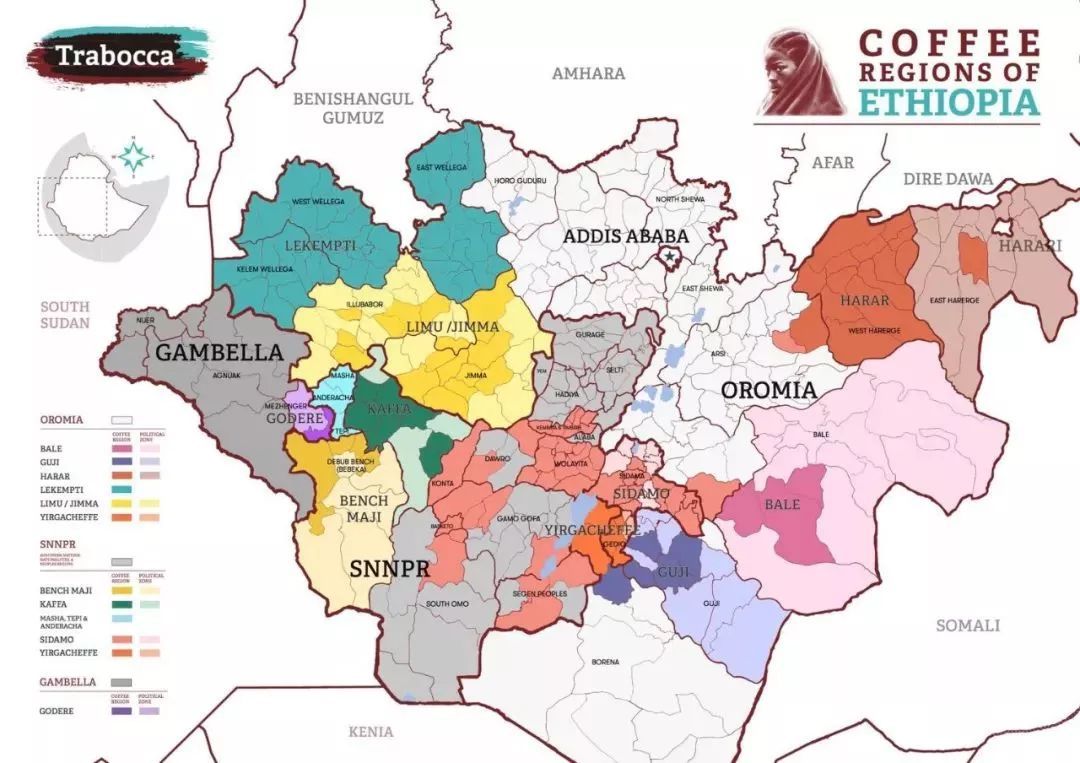
What is the Red Cherry Project? The most accurate coffee map of Ethiopia!
Professional coffee knowledge exchange more coffee bean information Please follow the coffee workshop (Wechat official account cafe_style) We often hear about the red cherry project, Ethiopia is the birthplace of coffee beans, the development rate of local tree species is very low, so we usually call Ethiopian tree species native species, because we have no idea how many coffee trees this piece of land is made of.
- Next

Jiamoni, Kenya-the story of a 72-hour coffee bean wash
Professional coffee knowledge exchange more coffee bean information please follow the coffee workshop (Wechat official account cafe_style) [Kenya Jiamoni exquisite 72-hour washing] country: Kenya region: Kilinaga Kirinyaga altitude: 1700-1800m soil: volcanic soil species: sl-28,sl-34 treatment: 72 hours washing 01 | the processing plant introduces Kiangombe workers.
Related
- Detailed explanation of Jadeite planting Land in Panamanian Jadeite Manor introduction to the grading system of Jadeite competitive bidding, Red bid, Green bid and Rose Summer
- Story of Coffee planting in Brenka region of Costa Rica Stonehenge Manor anaerobic heavy honey treatment of flavor mouth
- What's on the barrel of Blue Mountain Coffee beans?
- Can American coffee also pull flowers? How to use hot American style to pull out a good-looking pattern?
- Can you make a cold extract with coffee beans? What is the right proportion for cold-extracted coffee formula?
- Indonesian PWN Gold Mandrine Coffee Origin Features Flavor How to Chong? Mandolin coffee is American.
- A brief introduction to the flavor characteristics of Brazilian yellow bourbon coffee beans
- What is the effect of different water quality on the flavor of cold-extracted coffee? What kind of water is best for brewing coffee?
- Why do you think of Rose Summer whenever you mention Panamanian coffee?
- Introduction to the characteristics of authentic blue mountain coffee bean producing areas? What is the CIB Coffee Authority in Jamaica?

