What is the flavor of deep-roasted coffee? what is the flavor of deep-roasted coffee beans? deep-roasted Mantenin Italian mixed beans commercial coffee
Mellow creates a deep roasted fragrance

Since 1962, two Nobel laureates, Reichstein and Stottinger, pointed out that uran, mercaptan, and alkylpyrazine. In fact, scientists have made great progress in this area over the years after Diketone is the key to creating the aroma of coffee. Scholars such as Thomas Palerman (Thomas H Parliment), a chemist from the world-renowned Kraft Food Company (Kraft Foods), and Hall Howard D.Stahl, as well as David Rowe of Oxford Chenicals Chemical Company in the UK, all point out the charming aroma of coffee. Whether shallow baked, medium baked or re-baked, as long as it comes from mercaptan compounds, that is, mercaptan (Mercaptan or Thiol) and "chaff", "ethanol", "ketones" or "aldehydes" derived from aromatic compounds.
However, coffee does not contain such compounds, mercaptan compounds are the products of burning fire, which will increase with the deepening of baking depth. the formation of mercaptan compounds runs counter to the sour aroma that is afraid of fire, and the concentration of mercaptan in deep roasting is significantly higher than that in shallow roasting. This is why the cooked beans that enter the second explosion will be more mellow and charming than the coffee that is not over yet, and more generally accepted by the general public.
When the coffee bag is opened or ground into powder, the intoxicating aroma of the wine is wafted, mainly from mercaptan, as a criterion to determine whether the coffee meets the fresh standard.
The history of mercaptan is revealed.
According to the study of Palerman and Stowe in Coffee Flavor Chemistry, the precursors of mercaptan compounds are divided into "arabinogalactan" (Arabinogalactan) and "cysteine" (Cysteine) are stored in the thick cell walls of coffee beans in cellulose (Cellulose) and lignin (Lignin).
Furfuruyl, one of the raw materials for the synthesis of mercaptan, is the degradation product of arabinogalactan. The second primary "sulfur" of mercaptan compounds comes from the pyrolysis product of "cysteine". In other words. Mercaptan compounds are cellulose in the cell wall of coffee beans. To put it bluntly, the aromatic products of the degradation and polymerization of lignin and amino acids are the Mena reaction of sugars and amino acids, and mercaptan can also be said to be a polymer of "aldehydes and ketones" and "sulfur".
Most people see that the word "sulfur" is associated with the bad smell of sulfur and rotten eggs, but when sulfur is combined with urans and aldehydes and ketones, it creates a charming fragrance, which is the top secret of the strong aroma of coffee.
Perlerman and Stone pointed out in the sulfides in Food (The Sulfur Compounds inFoods) published by the American Chemical Society (AmericanChemical Society) that mercaptan increases with the roasting degree of coffee. According to the results of gas chromatography, the mercaptan of very shallow roasted coffee (Agtrron#95) soared to less than 10, 000 units of mercaptan (Agtrron#55) to 70, 000 units of mercaptan in deep roasting (Agtrron#32).
The replication of mercaptan with "carbohydrate" and "cysteine" in the laboratory requires higher energy to produce mercaptan, which coincides with the fact that the content of mercaptan in medium-deep baking is much higher than that in shallow baking, indicating that mercaptan production requires higher energy.
Mercaptan with chocolate, cream sugar, cocoa, banana, egg, and even meat aroma, is the contribution of light, medium and deep roasted coffee. In addition, there are three compounds closely related to the aroma of coffee, including "4-ethyl guaiacol" (4-ethylguaiacol), "alkylpyrazine" (Alkylpyrazines) and "isopentenyl mercaptan", which are also related to the thick cell wall of coffee (Prenyl mercaptan). The cellulose of coffee beans accounts for 40% of the bean weight, which makes the cell wall structure of coffee much harder and thicker than other seeds.
Palerman and Stowe are in "Why does coffee smell so good?" The conclusion of WhatMake That Coffee Smell So Good? points out that previous studies have shown that the precursors of mercaptan compounds may be hidden in the thick, hard cell walls of coffee, a finding that provides part of the answer to the question of why coffee is more mellow than other bell seeds or beans.
Don't try deep baking.
Try to bake soybeans, black beans, cashew nuts, sweet apricots and pumpkin seeds with a drum roaster. It is found that these beans or nuts have soft texture and are not resistant to heat. Although they also have fragrance, they are far worse than coffee. In the past, coffee chemists focused on the precursor aromatics such as sucrose, fat, protein and trigonelline in coffee, but in recent years they have turned to the long-neglected structural problems of coffee cell walls and polysaccharides. How to improve the thickness of coffee cell wall and the flavor of roasted coffee through post-processing of biochemical and planting technology will be an important topic.
Although the above studies have pointed out that mercaptan increases with the deepening of baking degree, do not misinterpret it as the more coffee is roasted, knowing that roasting is an extremely complex degradation and polymerization of thousands of compounds, although mercaptan increases with the baking degree. However, the "carbonized coke choking particles" and the bitter degradation products of "chloric acid" accumulated by deep roasting also increase rapidly, which is easy to mask or counteract the aroma of mercaptan.
If the deep baking or re-baking technology is only proficient, do not try, it is best to use the dense explosion at the beginning or middle of the second explosion to come out of the oven quickly, so as not to outweigh the loss, because the baker who has the ability to control the carbonization degree and coke choking smell of the second explosion tail to the lowest, it's quite rare.
Important Notice :
前街咖啡 FrontStreet Coffee has moved to new addredd:
FrontStreet Coffee Address: 315,Donghua East Road,GuangZhou
Tel:020 38364473
- Prev
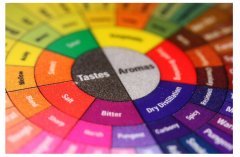
Coffee aroma mixed pine aroma coffee flavor round SCAA boutique coffee
Americans often say: One mans meat is another mans poison, that is, one person is regarded as delicious, others as poison. The aroma spectrum of dry distillation can be divided into three categories: resin rhyme, spicy rhyme and carbonized rhyme, as follows: 1. Resin rhyme: turpentine and choking turpentine: turpentine, chicory, mahogany, wine, blackcurrant branches and leaves: Rosemary, eucalyptus alcohol, Eucalyptus leaves,
- Next

Make concentrated espresso Italian style, Blue Mountain flavor and Asian mellow coffee beans.
When you make Espresso, you only have about 30 seconds to extract, which means that those large coffee particles are actually useless fillers that cannot be extracted thoroughly or evenly.
Related
- Beginners will see the "Coffee pull flower" guide!
- What is the difference between ice blog purified milk and ordinary milk coffee?
- Why is the Philippines the largest producer of crops in Liberia?
- For coffee extraction, should the fine powder be retained?
- How does extracted espresso fill pressed powder? How much strength does it take to press the powder?
- How to make jasmine cold extract coffee? Is the jasmine + latte good?
- Will this little toy really make the coffee taste better? How does Lily Drip affect coffee extraction?
- Will the action of slapping the filter cup also affect coffee extraction?
- What's the difference between powder-to-water ratio and powder-to-liquid ratio?
- What is the Ethiopian local species? What does it have to do with Heirloom native species?

